Chem 6AL Gainer IR Spectroscopy
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
46 Terms
IR spectroscopy
- can be used to determine the functional group of a molecule
- uses IR light to bend bonds and determine their absorption spectra
bond bending vs. bond stretching
when a bond stretches, the bond length changes
- seen in higher energy portion of the IR spec (i.e. 4000-1400)
when a bond bends, the bond angle changes
- seen in lower energy portion of the IR spec (i.e. 1400-400)
IR spec graph (x vs. y axes)
- x-axis = wavelength / wavenumbers (cm^-1)
- y-axis = amount of light absorbed; more light absorbed = stronger "peak" (dip in actuality)
strong vs. weak intensity absorption bands
describes the depth of an absorption band
- strong = large dip, weak = small dip
broad vs. sharp intensity absorption bands
describes the width of absorption band
- broad = wide band, sharp = narrow band
what factors affect the intensity of an absorption band
- the dipole moment of a bond
- the number of bonds present
- the concentration of a sample
bond dipole moment effect on IR spectra
- as a bond stretches, the dipole moment increases
- stronger dipole moment / higher electronegativity = absorption of higher freq. light
- O-H > N-H > C-H
bond number effect on IR spectra
- the higher quantity of a certain type of bond, the more light it will absorb
- causes a deeper peak
sample concentration effect on IR spectra
an overconcentrated sample can absorb too much light and give an unusable IR spectra
what three factors affect the position of an absorption band?
- the atomic mass of an atom
- the bond order/strength between two atoms
- resonance in an atom
atomic mass effect on IR spectra (hooke's law)
- heavier atoms cause bonds to vibrate slower and absorb light at lower spectra
- lighter atoms vibrate faster and absorb light at higher spectra
bond order/strength effect on IR spectra
- stronger bonds cause atoms to be held tighter together
- i.e. C=C > C-C
resonance effect on IR spectra (delocalization vs. stabilization)
- resonance delocalizes electrons and creates partial charges, will weaken bonds in comparison to bond order
- i.e. ketone = 1720, w/ double bond resonance = 1680
- resonance can also stabilize certain bonds
- i.e. amide = 1680, ester = 1740 b/c esters aren't as stable with a positive charge as amides
IR Spectrum Range of Functional Group Bond:
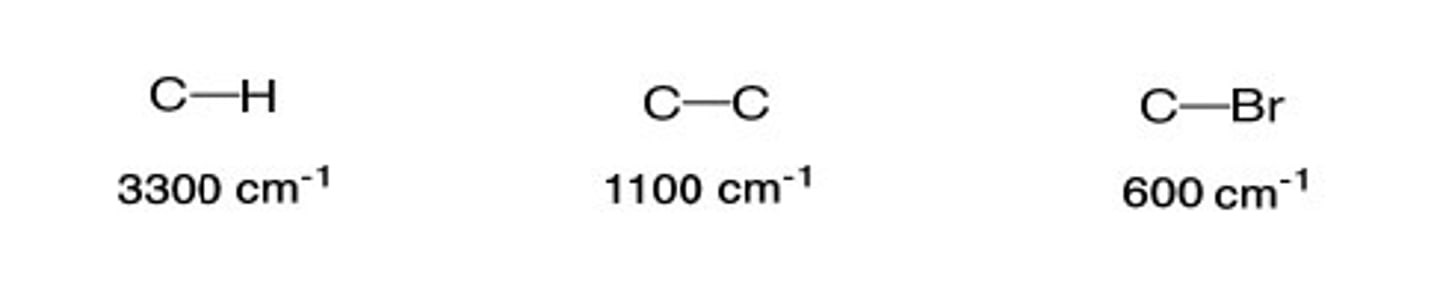
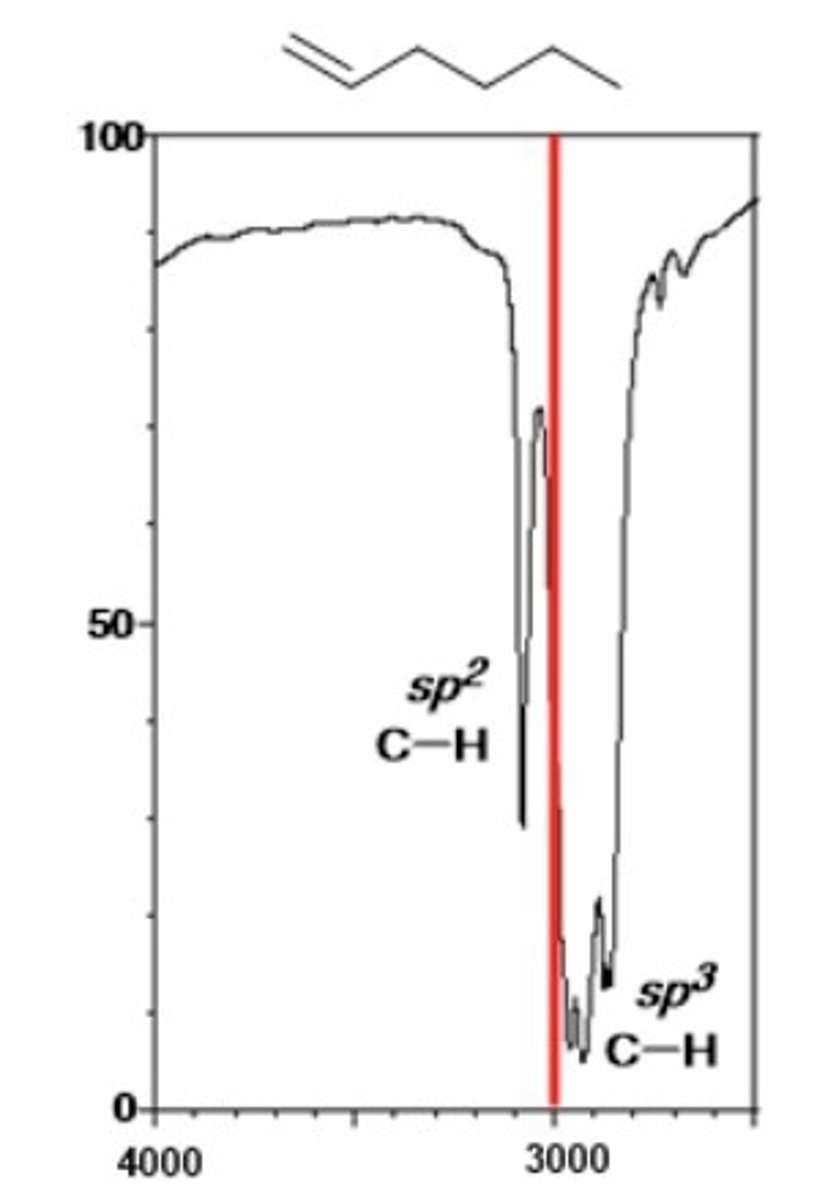
1/2. Alkene C-H (sp^2)
Shape? Intensity?
3000-3100 cm-1
Shape: sharp
Intensity: medium
IR Spectrum Range of Functional Group Bond:
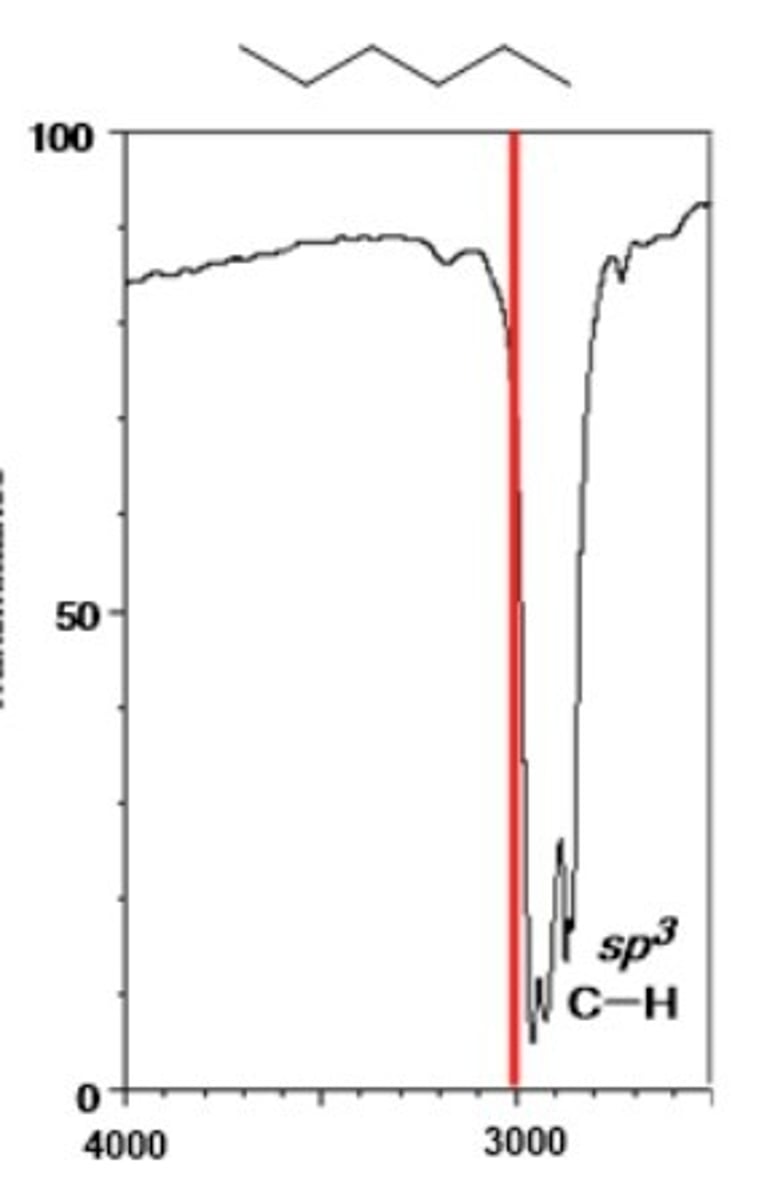
alkane C-H (sp^3)
Shape? Intensity?
2850-3000 cm-1
Shape: often strong
IR Spectrum Range of Functional Group Bond:
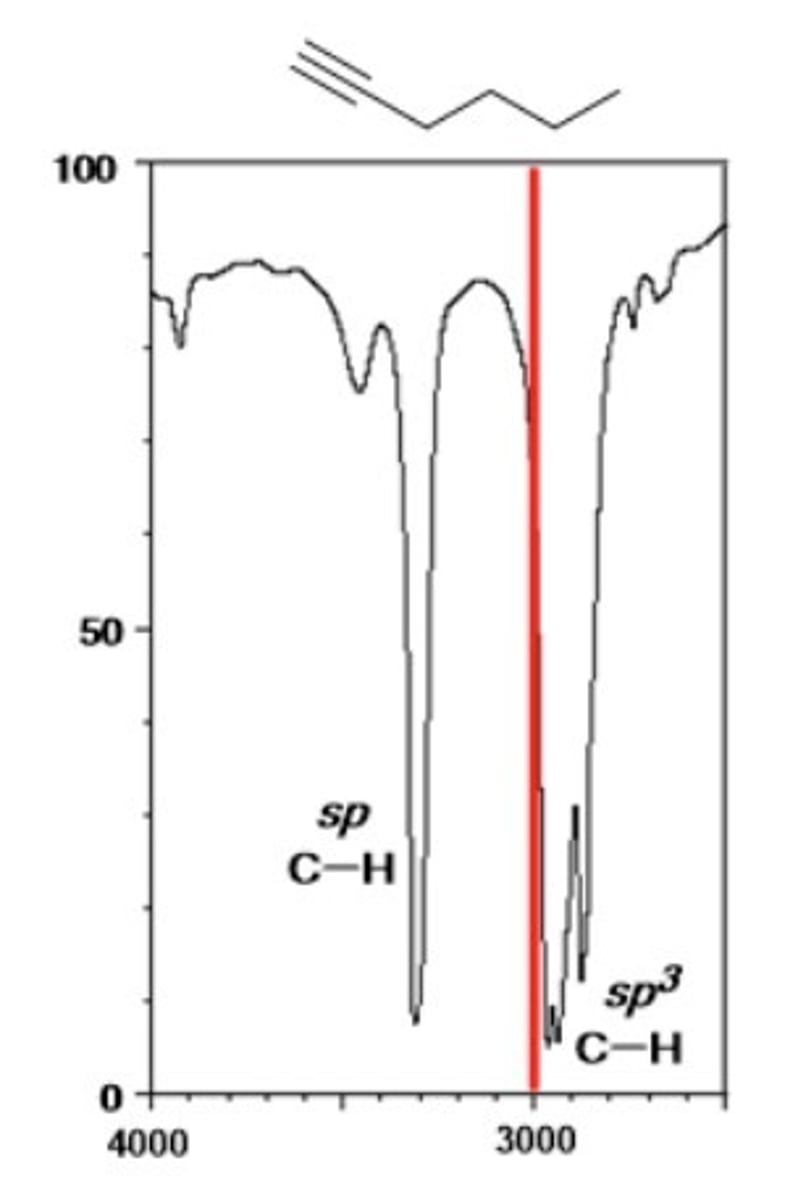
1/2. alkyne C-H (sp)
Shape? Intensity?
3300 cm-1
Shape: sharp
Intensity: medium
IR Spectrum Range of Functional Group Bond:
2/2. Alkene C=C
Shape? Intensity?
1600-1650 cm-1
Shape: sharp
Intensity: medium
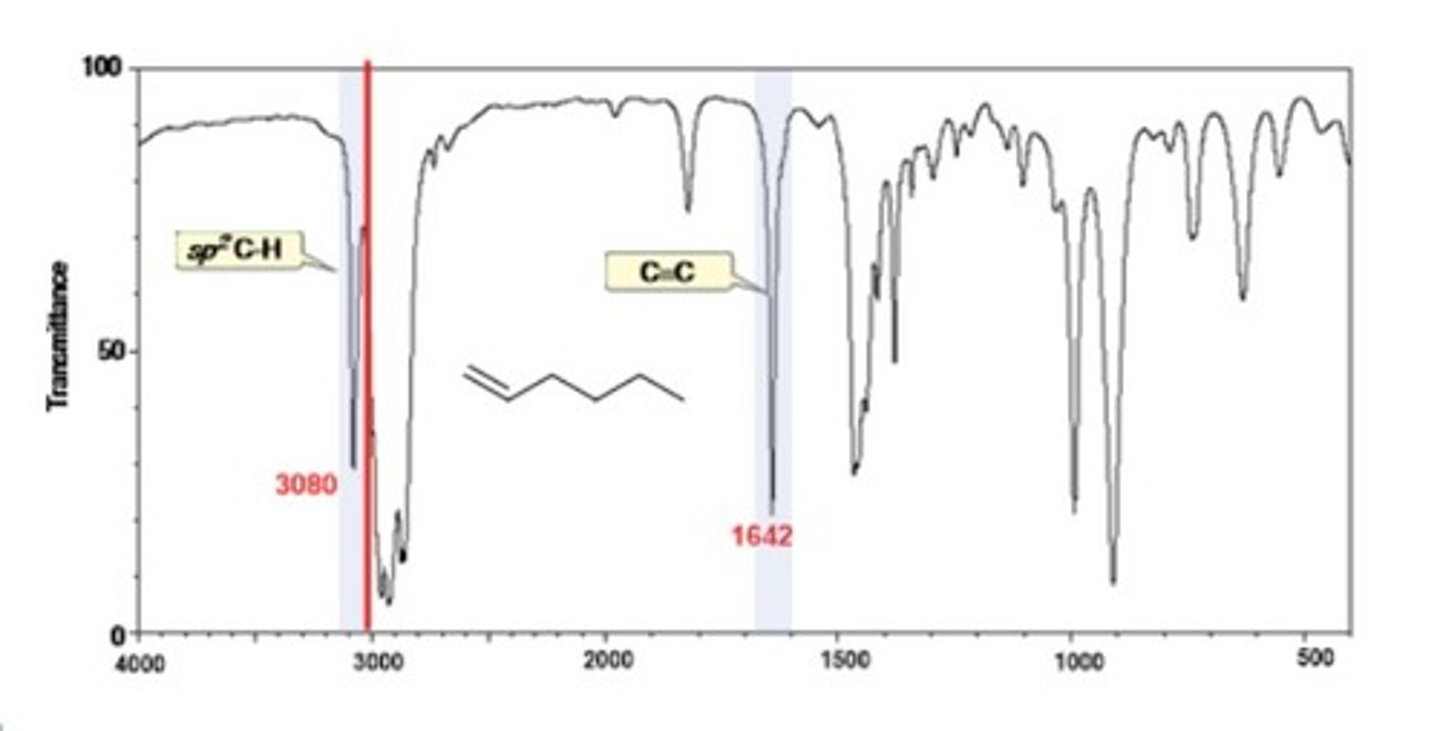
IR Spectrum Range of Functional Group Bond:
aromatic C=C
Shape? Intensity?
1475-1600 cm-1
Shape: sharp
Intensity: medium
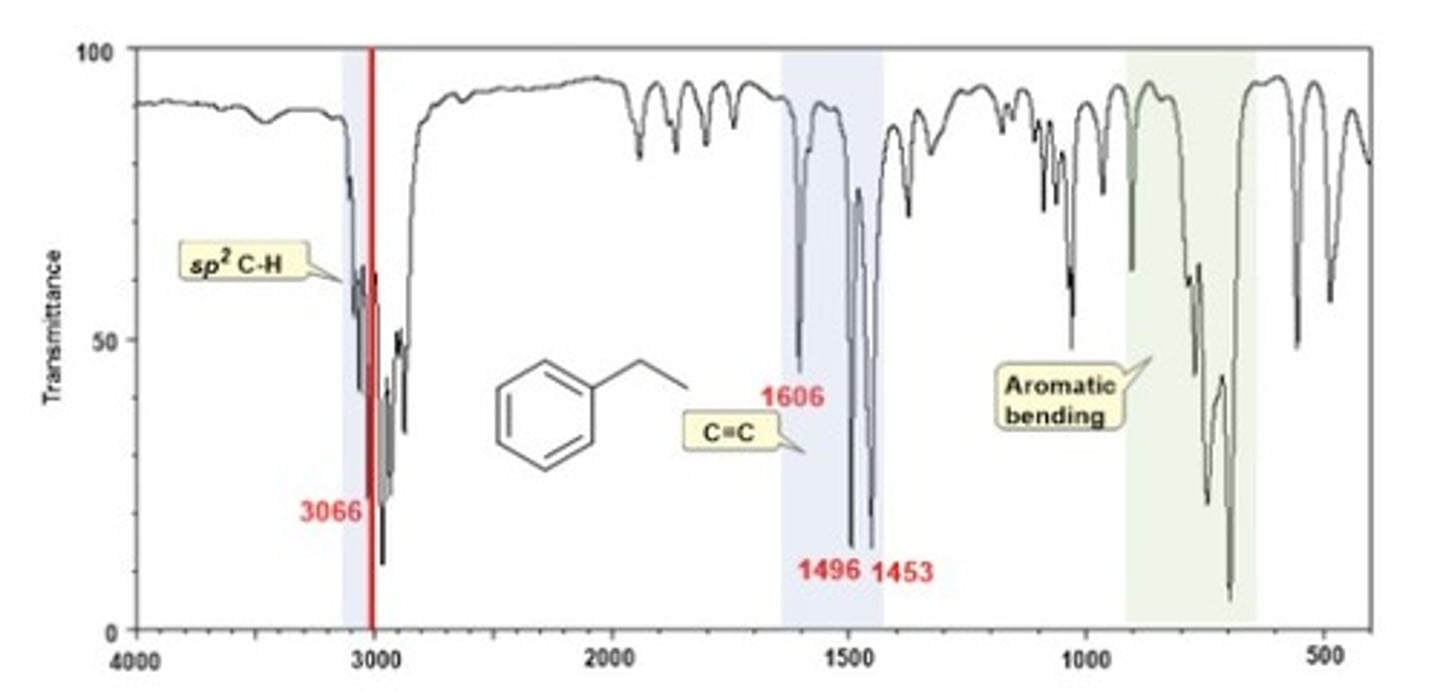
IR Spectrum Range of Functional Group Bond:
Imine C=N
Shape? Intensity?
1550-1650 cm-1
Shape: sharp
Intensity: medium
IR Spectrum Range of Functional Group Bond:
2/2. alkyne C=C
Shape? Intensity?
2100-2200 cm-1
Shape: sharp
Intensity: medium-weak
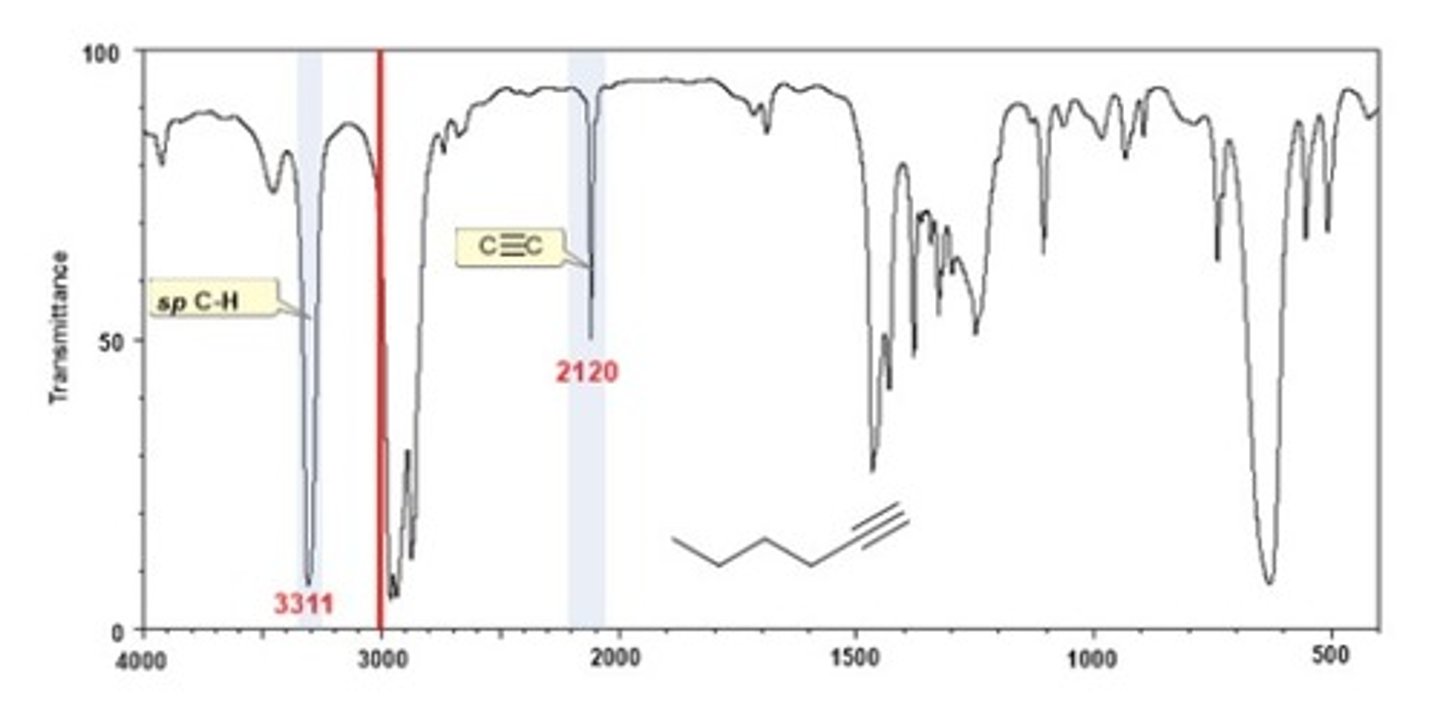
IR Spectrum Range of Functional Group Bond:
Nitrile C=N
Shape? Intensity?
2200-2300 cm-1
Shape: broad
Intensity: medium
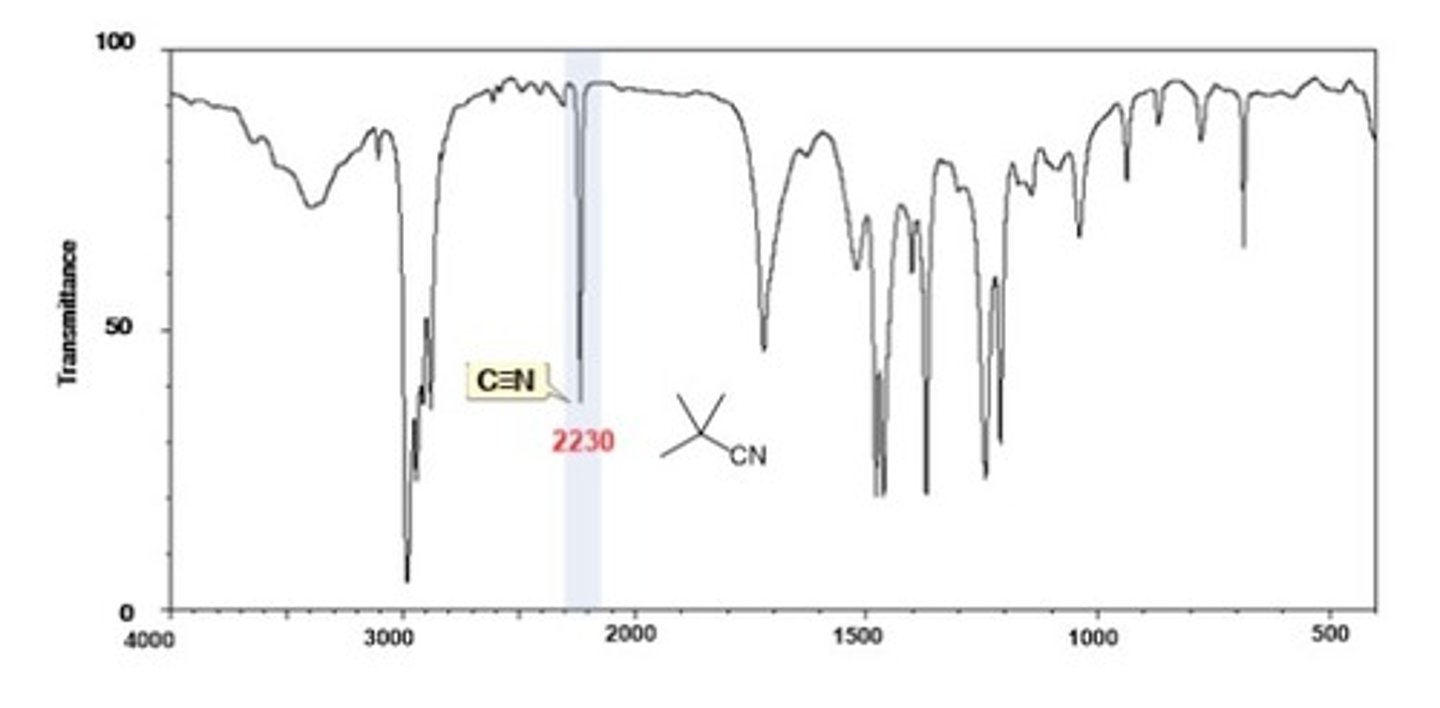
IR Spectrum Range of Functional Group Bond:
Alcohol O-H
Shape? Intensity?
3300 cm-1
Shape: broad, smooth
Intensity: strong
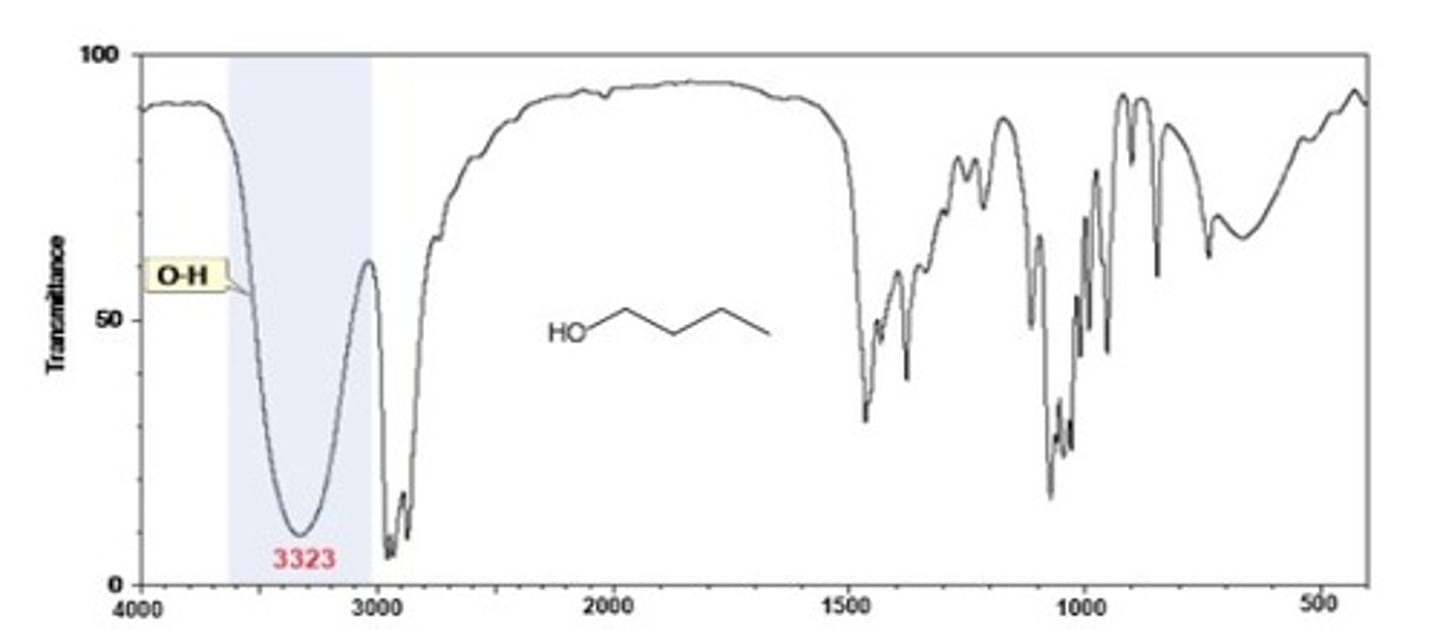
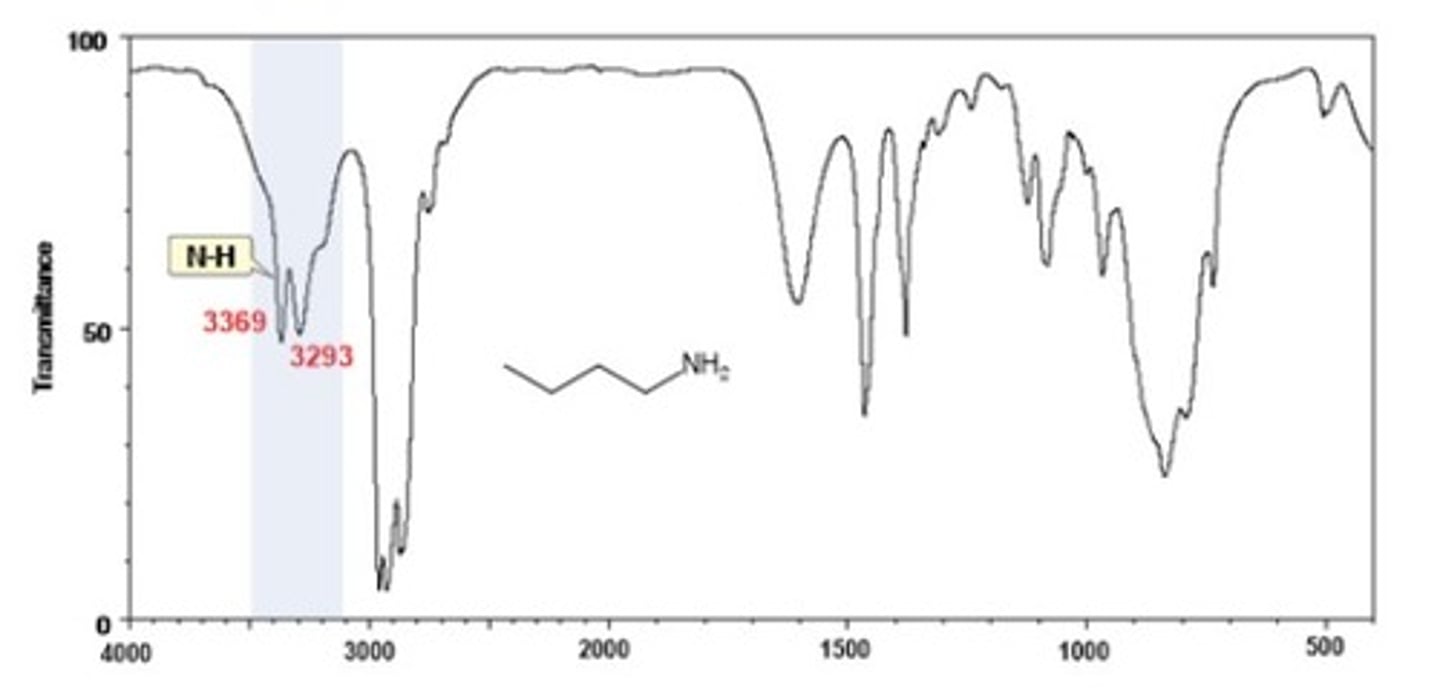
IR Spectrum Range of Functional Group Bond:
Amine N-H
Shape? Intensity?
3300 cm-1
Shape: medium
Intensity: broad
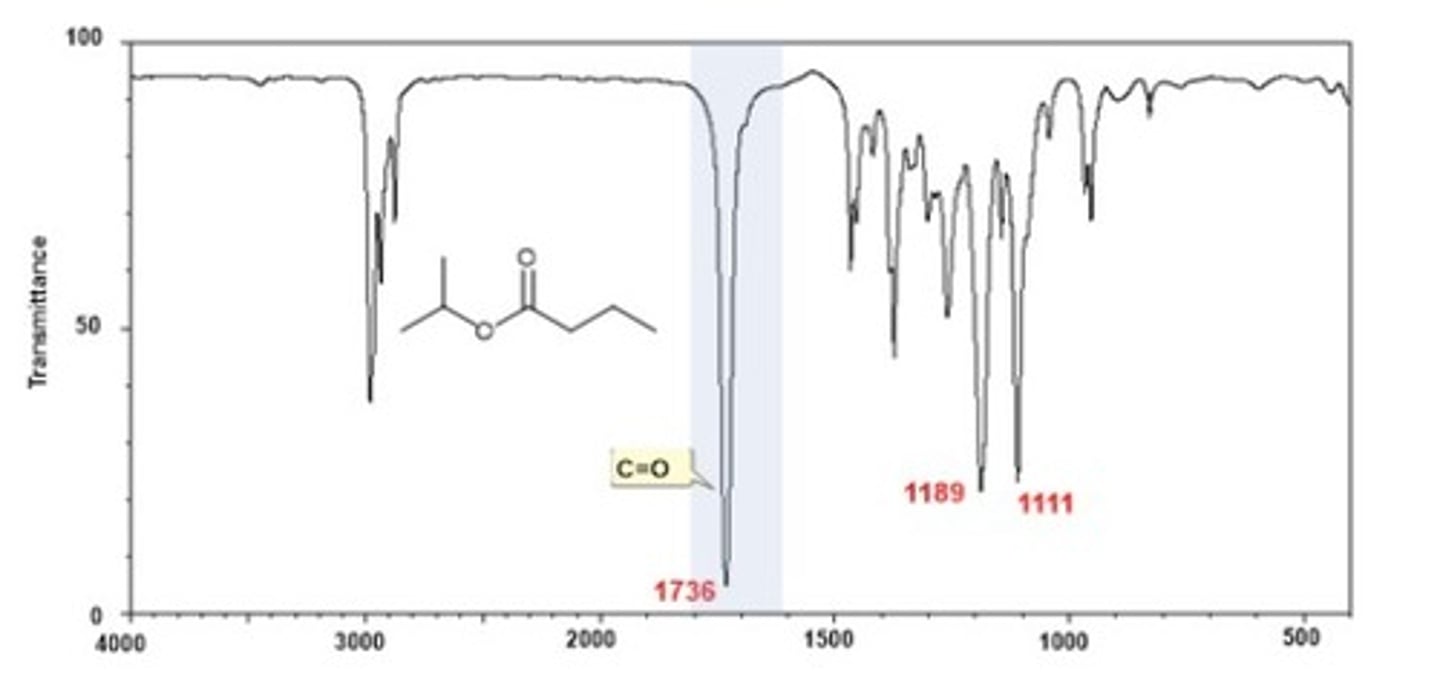
IR Spectrum Range of Functional Group Bond:
1/2. Ester C=O
Shape? Intensity?
1740 cm-1
Shape:
Intensity: strong
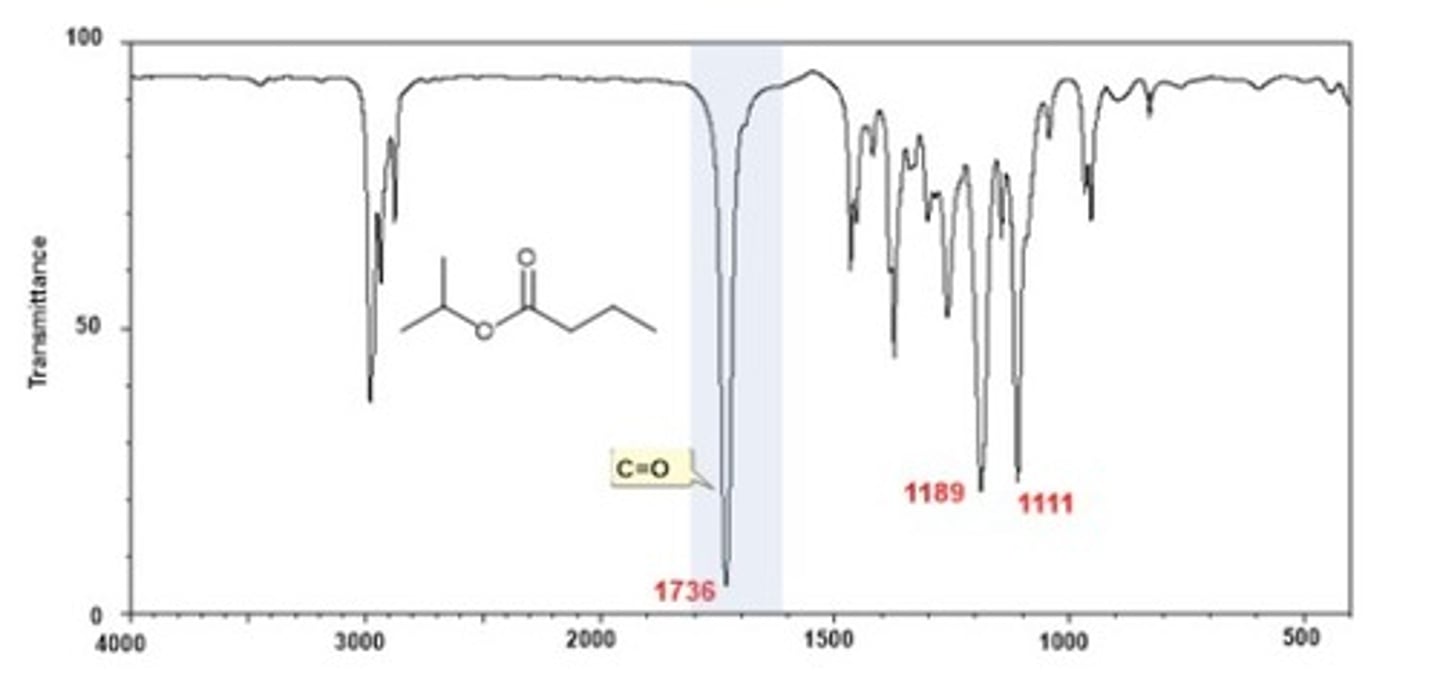
IR Spectrum Range of Functional Group Bond:
2/2. Ester C-O
Shape? Intensity?
1000-1300 cm-1
Shape: Often two bands
Intensity: strong
IR Spectrum Range of Functional Group Bond:
2/2. Ether C-O
Shape? Intensity?
1050 cm-1
Shape: One band
Intensity: strong
IR Spectrum Range of Functional Group Bond:
1/2. aldehyde C=O
Shape? Intensity?
1730 cm-1
Shape: strong
Intensity: -
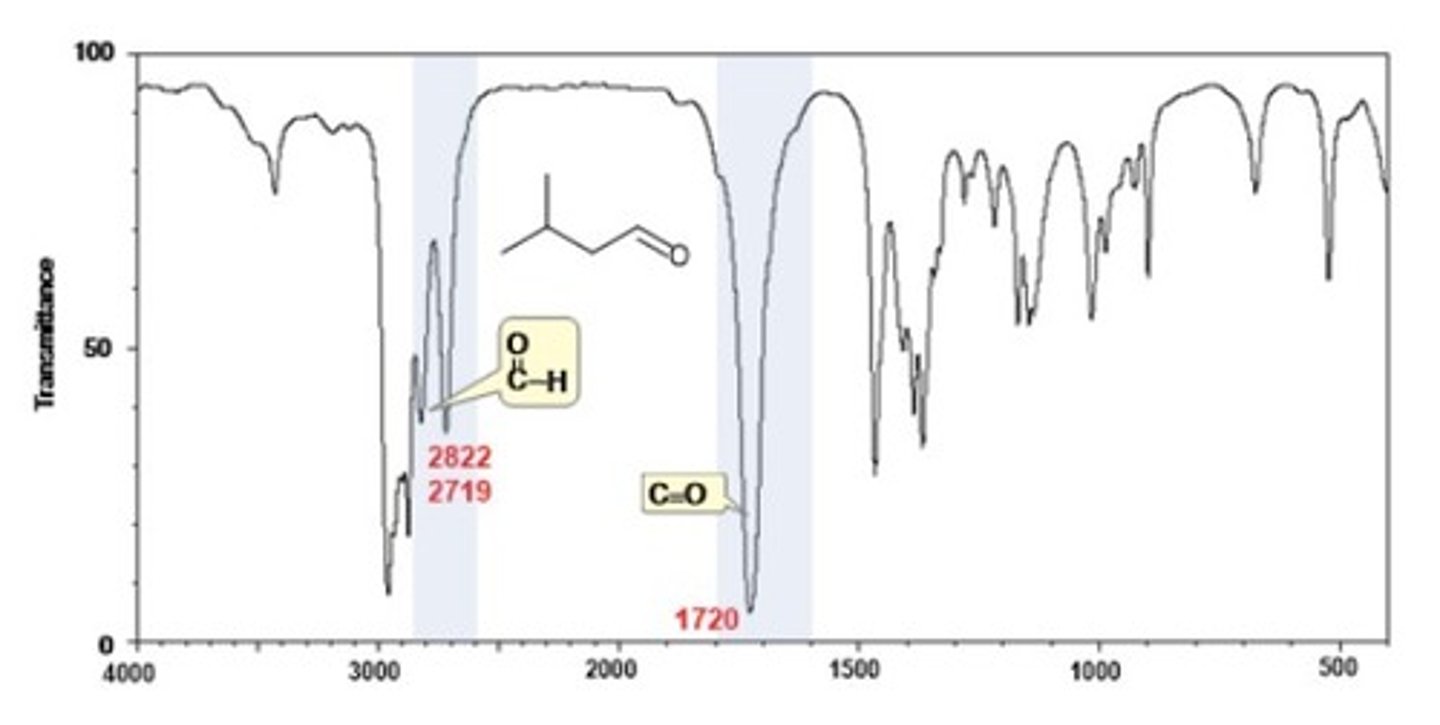
IR Spectrum Range of Functional Group Bond:
2/2. aldehyde H-CO
Shape? Intensity?
2700, 2800 cm-1
Shape: medium
Intensity: -
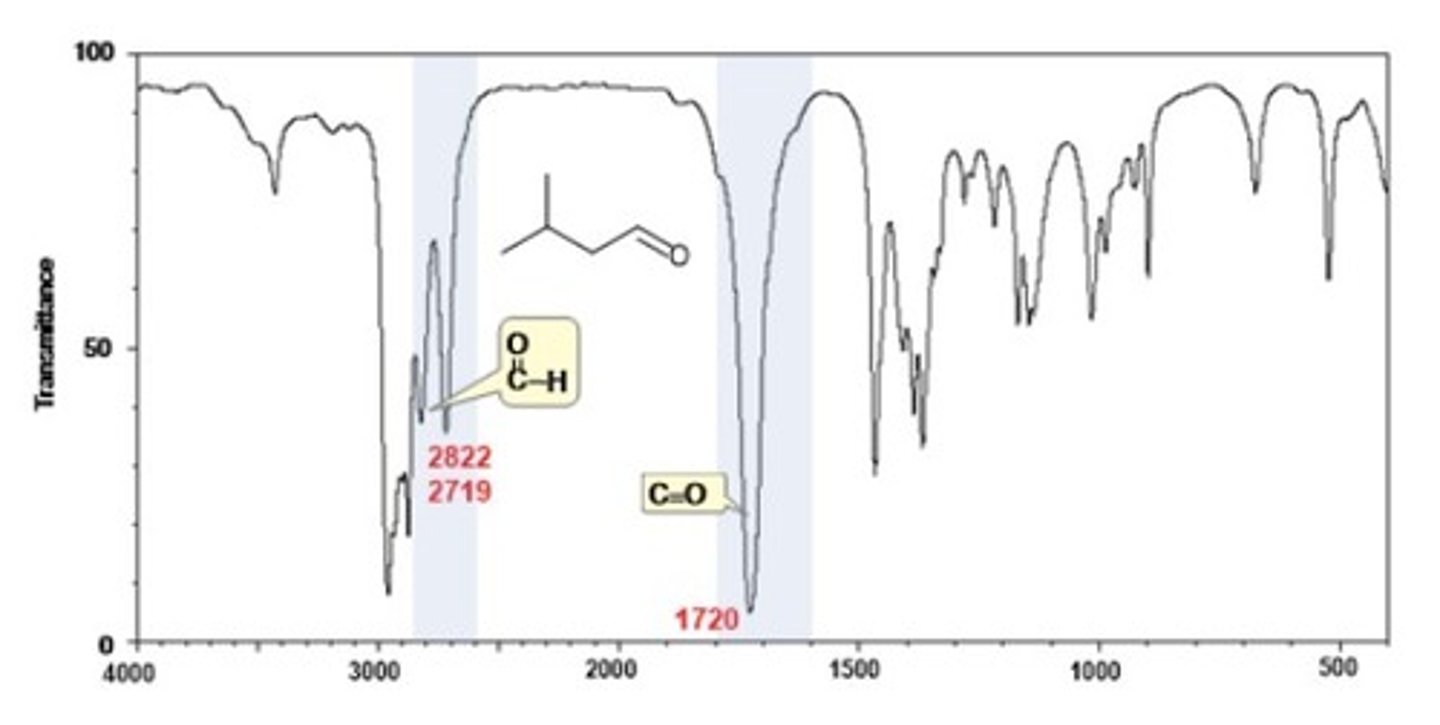
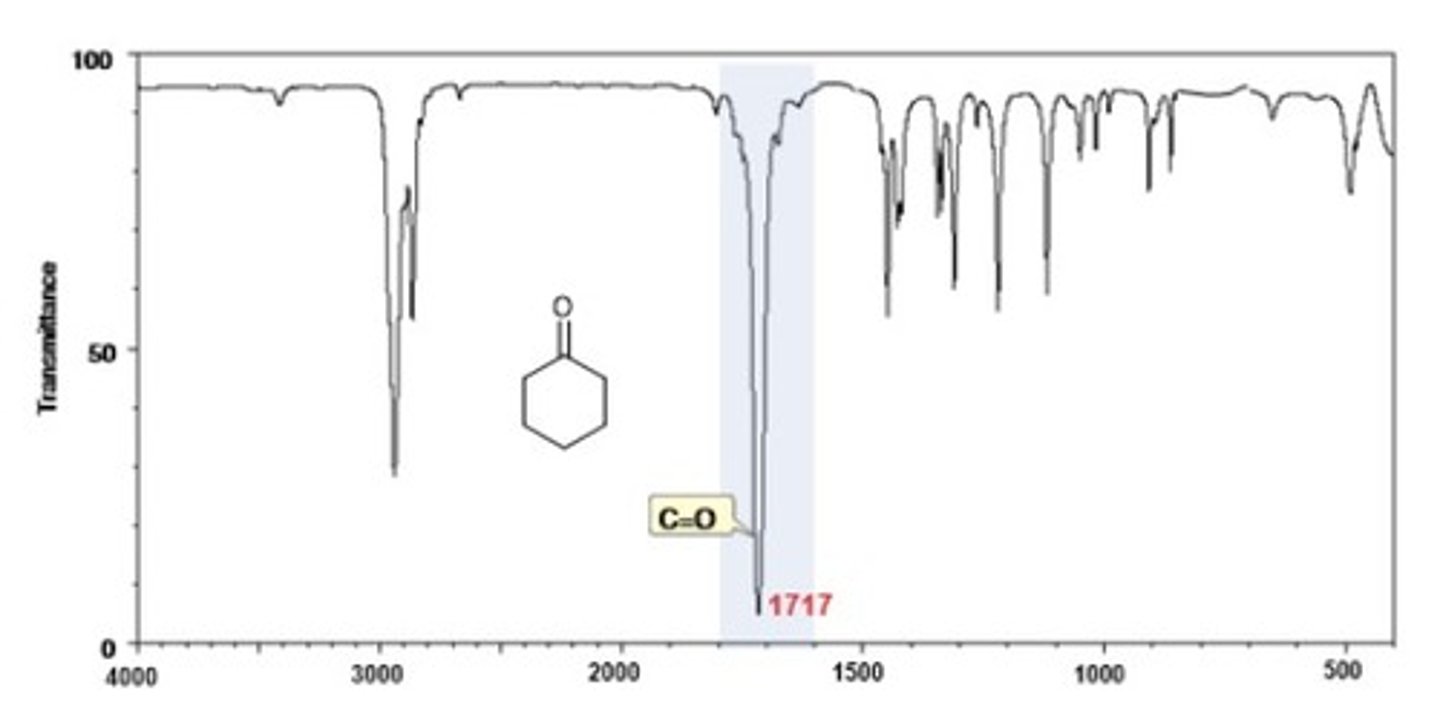
IR Spectrum Range of Functional Group Bond:
ketone C=O
Shape? Intensity?
1720 cm-1
Shape: strong
Intensity: -
IR Spectrum Range of Functional Group Bond:
1/2. carboxylic acid C=O
Shape? Intensity?
1710 cm-1
Shape: strong
Intensity: -
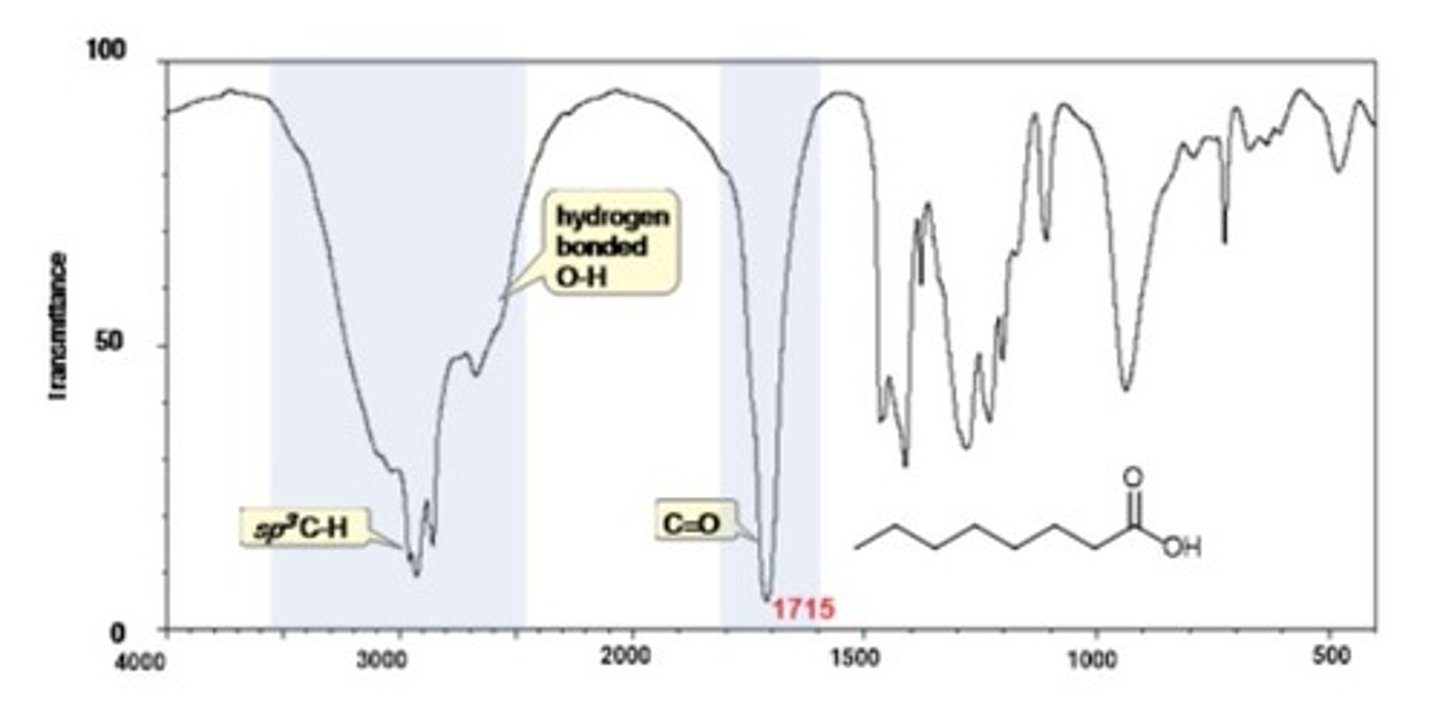
IR Spectrum Range of Functional Group Bond:
1/2. carboxylic acid C-O
Shape? Intensity?
1250 cm-1
Shape: strong
Intensity: -
IR Spectrum Range of Functional Group Bond:
2/2. carboxylic acid O-H
Shape? Intensity?
2500-3500 cm-1
Shape: jagged
Intensity: very broad
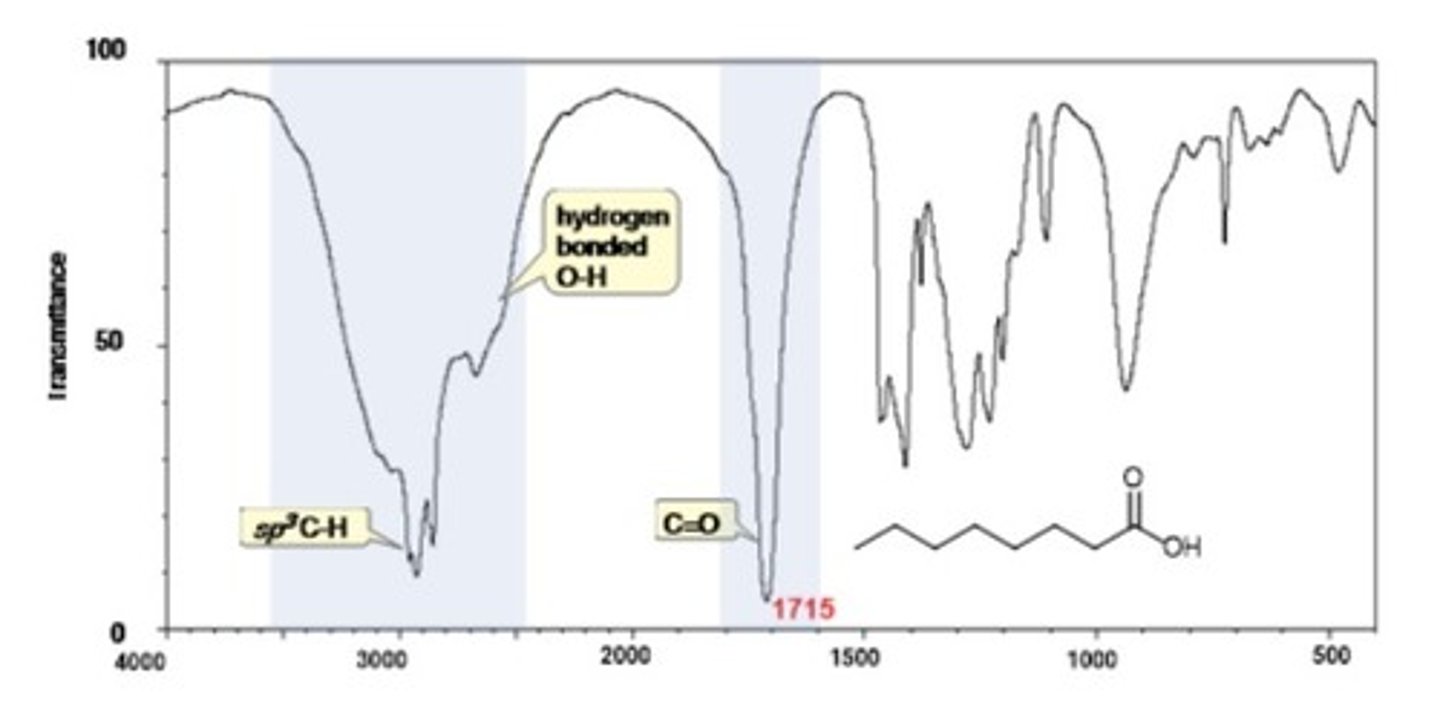
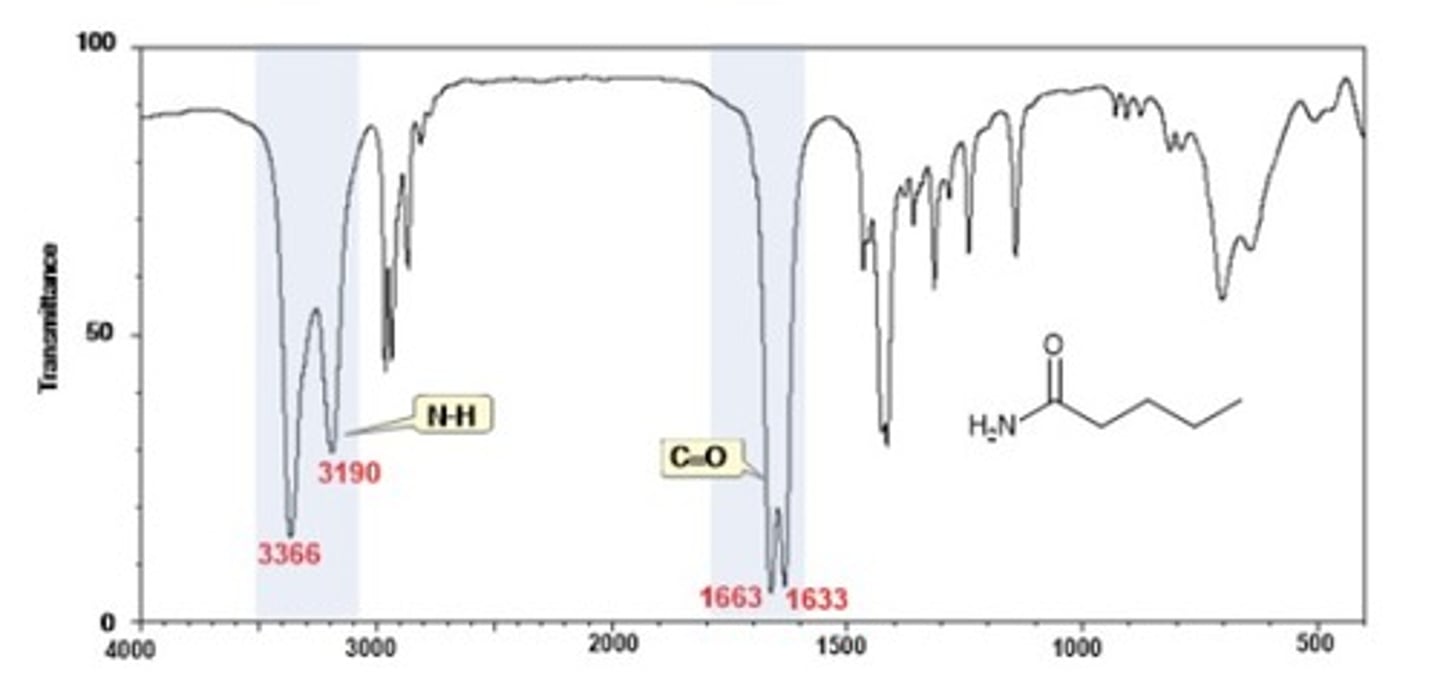
IR Spectrum Range of Functional Group Bond:
1/2. amide C=O
Shape? Intensity?
1650 cm-1
Shape: broad
Intensity: strong
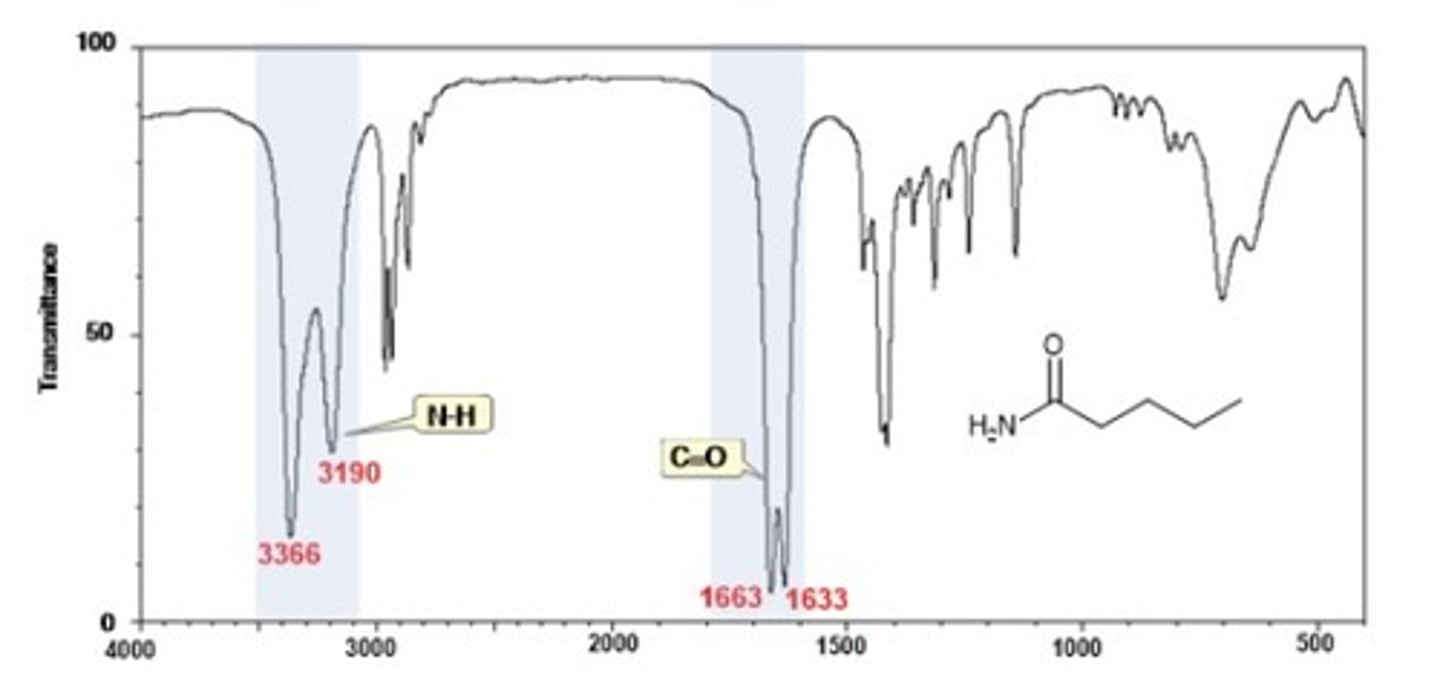
IR Spectrum Range of Functional Group Bond:
2/2. amide N-H
Shape? Intensity?
3300 cm-1
Shape: broad
Intensity: strong
IR Spectrum Range of Functional Group Bond:
1/2. alkyl halide C-Cl
Shape? Intensity?
600-800 cm-1
Shape: Strong
Intensity: Strong
IR Spectrum Range of Functional Group Bond:
2/2. alkyl halide C-Br
Shape? Intensity?
500- 600 cm-1
Shape: Strong
Intensity: Strong
IR Spectrum analysis steps (1-5)
1. Distinguish C-H stretches of alkanes, alkenes, and alkynes by their position relative to ________
3000 cm-1
IR Spectrum analysis steps (1-5)
2. A sharp peak at ____-____cm-1 suggests a C=C stretch
1600-1650 cm-1
IR Spectrum analysis steps (1-5)
3. Look at _____cm-1 for O-H and N-H stretches (carboxylic acid O-H stretches occur over a broader range of 2500-3500 cm-1)
3300 cm-1
IR Spectrum analysis steps (1-5)
4. Bands near _____ cm-1 suggest a triple bond
2200 cm-1
IR Spectrum analysis steps (1-5)
5. A strong band in the ____-____ cm-1 region indicates a carbonyl
1650-1800 cm-1
Key principle in IR:
The (a) ______ of IR bands in a spectrum often tells us just as much as their (b) _______.
a. absence
b. presence
IR diagnostic or characteristic region range
____-____ cm-1
4000-1500 cm-1
IR fingerprint region range:
____-____ cm-1
1500-400 cm-1
Hooke's Law: Bond strength is directly proportional to the frequency, so (a) _______ bonds vibrate (b) _______ and absorb IR light of (c) _______ frequency.
Bond strength is directly proportional to the frequency, so (a) stronger bonds vibrate (b) faster and absorb IR light of (c) higher frequency.
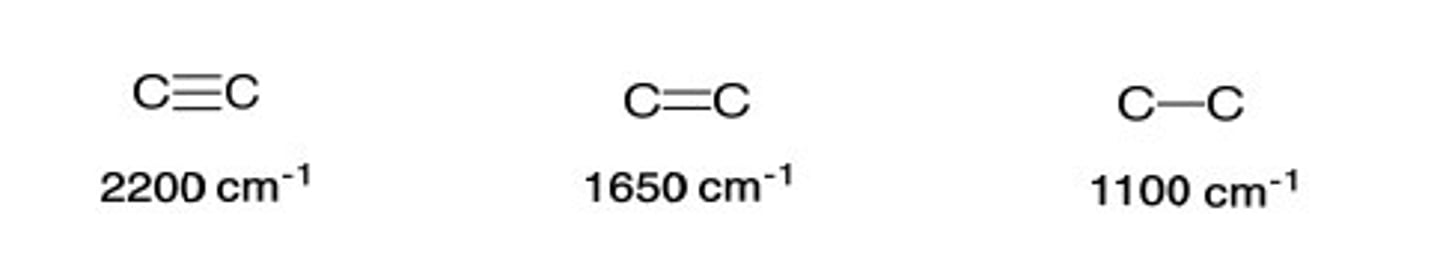
According to Hooke's Law, reduced mass is indirectly proportional to the frequency, so bonds with (a)_______ atoms vibrate (b)________ and absorb IR light of (c)________ frequency
According to Hooke's Law, reduced mass is indirectly proportional to the frequency, so bonds with (a) lighter atoms vibrate (b) faster and absorb IR light of (c) higher frequency