Valence Shell Electron Pair Repulsion Theory (copy)
0.0(0)
0.0(0)
Card Sorting
1/24
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
1
New cards
what are shapes defined by?
their bond angles
2
New cards
linear
180 degrees
3
New cards
trigonal planar
120 degrees
4
New cards
tetrahedral
109.5 degrees
5
New cards
How are electron pairs arranged?
they are arranged symmetrically with maximum separation since negative charges repel
6
New cards
if there are 2 electron pairs...
they are 180 degrees apart and have a linear geometry
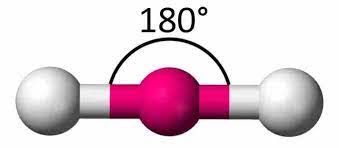
7
New cards
if there are 3 electron pairs...
they are 120 degrees apart and have a triangular planar geometry
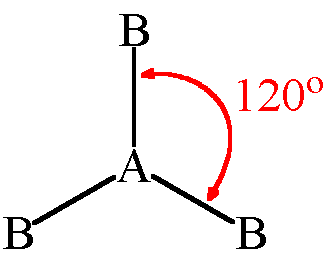
8
New cards
if there are 4 electron pairs...
they are 109.5 degrees apart and have a tetrahedral geometry
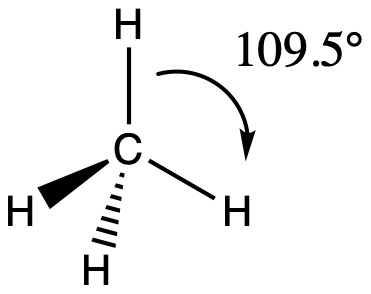
9
New cards
if there are 5 electron pairs...
angles of 90 and 120 degrees and have a trigonal bipyramidal (TBP) geometry
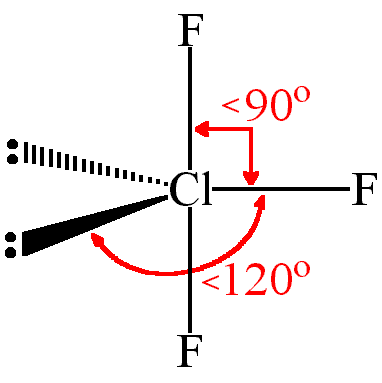
10
New cards
if there are 6 electron pairs...
they have 90 degree angles and have a octahedral geometry
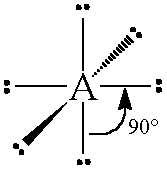
11
New cards
What is an electron pair of geometry
the arrangement of electron pairs around a central atom
12
New cards
what is molecular geometry
arrangement of atoms around a central atom
13
New cards
when all electrons pairs are bonding pairs...
electron pair of geometry = molecular geometry
14
New cards
when there are unshared or lone electron pairs...
electron pair of geometry does not equal molecular geometry
15
New cards
AXnEe notation values definitions
A = central atom
Xn = number of ligands (atoms) attached to the central atom
E = non-bonding electron pairs
e = number of lone eelctrons
Xn = number of ligands (atoms) attached to the central atom
E = non-bonding electron pairs
e = number of lone eelctrons
16
New cards
2 bonding pairs and 1 non-bonding
electron pair geometry: trigonal planar
molecular geometry: bent
angle: 104.5
molecular geometry: bent
angle: 104.5
17
New cards
3 bonding pairs and 1 non-bonding
electron pair geometry: tetrahedral
molecular geometry: trigonal planar
angle: 107
molecular geometry: trigonal planar
angle: 107
18
New cards
2 bonding with 2 non-bonding
electron pair geometry: tetrahedral
molecular geometry: bent
angle: 104.5
molecular geometry: bent
angle: 104.5
19
New cards
4 bonding with 1 non-bonding
electron pair geometry: trigonal bipyramidal
molecular geometry: seesaw
angle: 90 and 120
molecular geometry: seesaw
angle: 90 and 120
20
New cards
3 bonding with 2 non-bonding
electron pair geometry: trigonal bipyramidal
molecular geometry: T-shape
angle: 90
molecular geometry: T-shape
angle: 90
21
New cards
2 bonding with 3 non-bonding
electron pair geometry: trigonal bipyramidal
molecular geometry: linear
angle: 180
molecular geometry: linear
angle: 180
22
New cards
5 bonding with 1 non-bonding
electron pair geometry: octahedral
molecular geometry: square pyramid
angle: less than 90
molecular geometry: square pyramid
angle: less than 90
23
New cards
4 bonding 2 non-bonding
electron pair geometry: octahedral
molecular geometry: square planar
angle: 90
molecular geometry: square planar
angle: 90
24
New cards
3 bonding with 3 non-bonding
electron pair geometry: octahedral
molecular geometry: T-shape
angle: less than 90
molecular geometry: T-shape
angle: less than 90
25
New cards
2 bonding with 4 non-bonding
electron pair geometry: octahedral
molecular geometry: linear
angle: 180
molecular geometry: linear
angle: 180