Practical 8: Trypsin activity in earthworms
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
27 Terms
Enzymes increase rate of reaction by____________________
They create no changes in ____________ or in an enzyme making it a _________
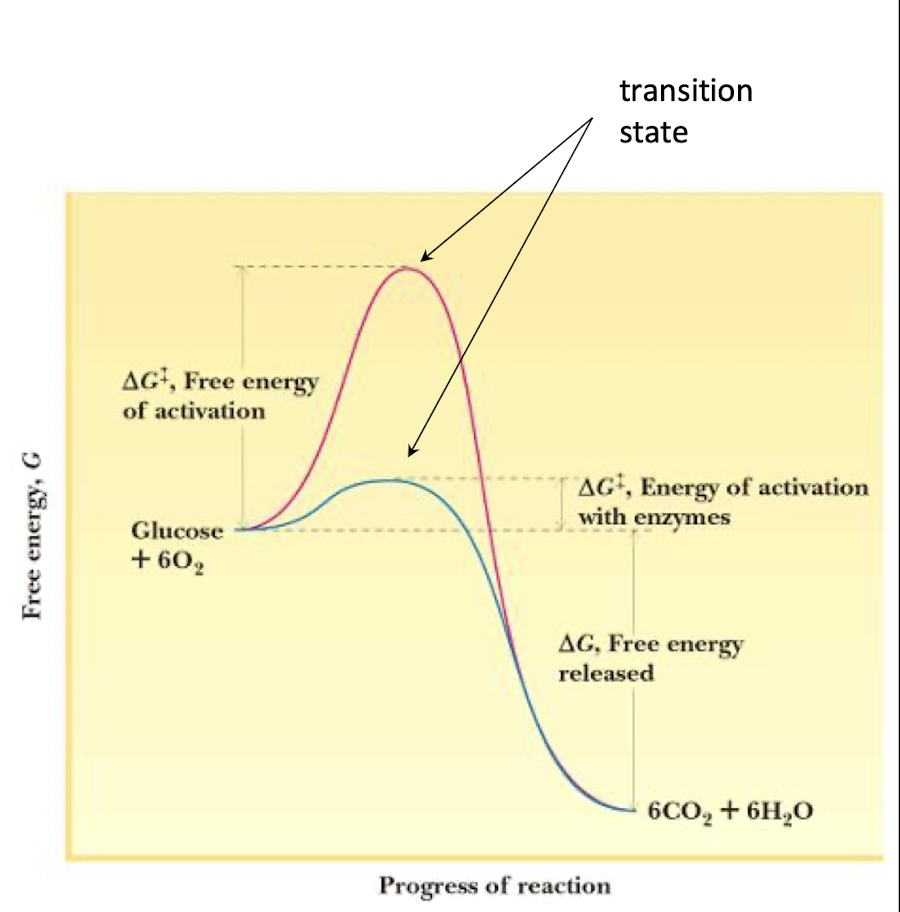
lowering the activation energy barrier (ΔG‡) , overall thermodynamics i.e. ΔG or in an enzyme, making it a true catalyst
what are examples of specific enzymes used in industry?
• Food – invertase
• Brewing – amylase
• Diagnostic kits - glucose oxidase
• Detergents - protease
• Textiles – protease & lipase

what are 3 reasons why enzyme assays are carried out?
To examine inhibition (i.e drug screening) and the effects of
inhibitors on enzyme activity e.g. the drug ritonavir inhibits HIV protease
To detect the levels of enzyme activity in tissues, extracts, purified proteins, industrial processes
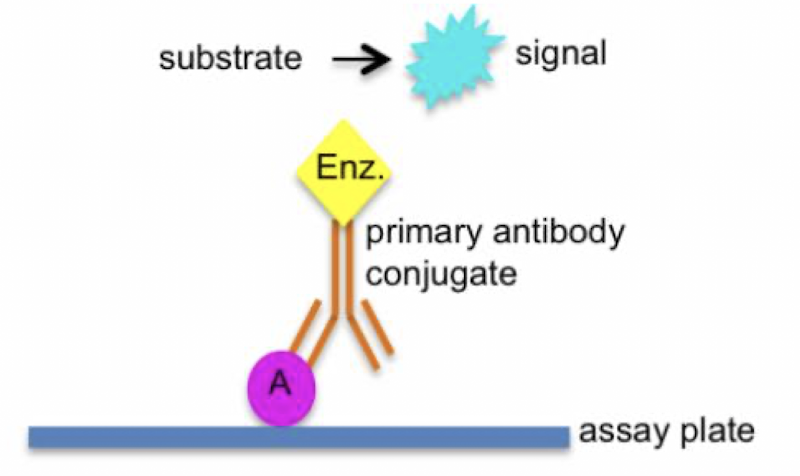
To detect biomolecules by linking enzymes to other moieties eg ELISA = Enzyme-linked immunosorbent assay, often used to detect bacteria in food.
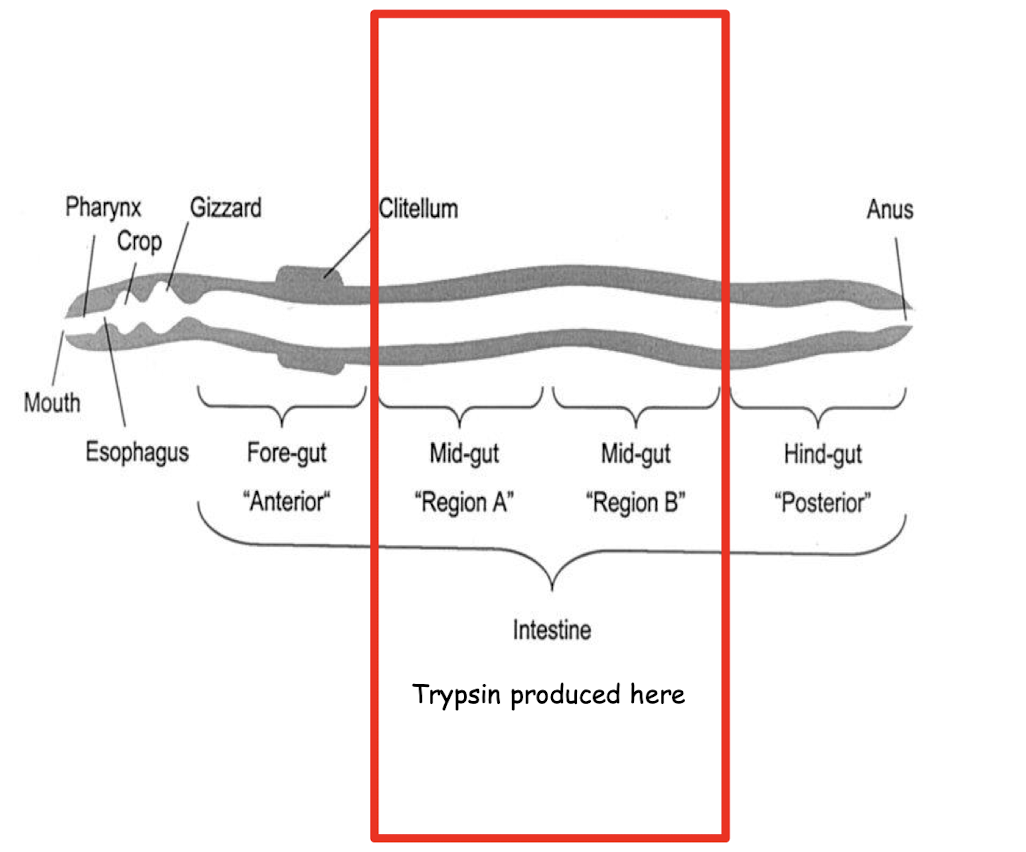
where is trypsin produced in earthworms?
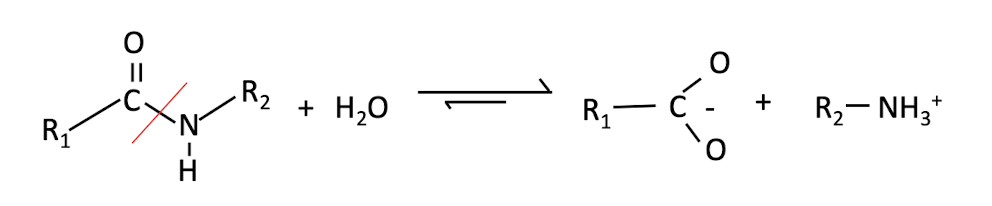
Trypsin is a _______ that cleaves proteins using a __________ reaction which is the addition of __________ , which is very ___
protease cleaves proteins using a hydrolysis reaction, addition of water to the peptide bond in proteins, is very slow
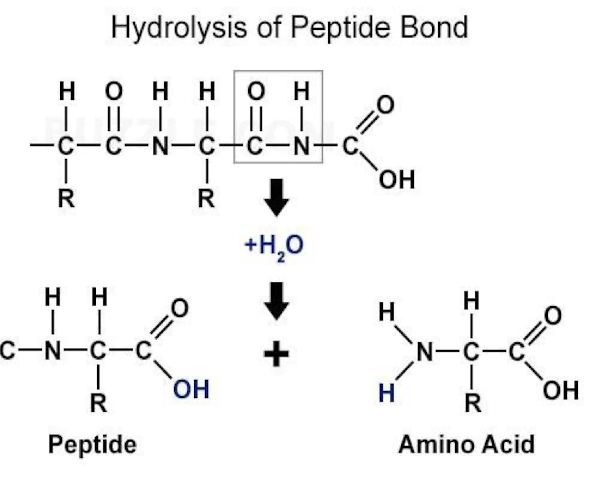
Why is hydrolysis of peptide bonds extremely slow?
• Absence of a catalyst half-life at neutral pH is b/w 10 -100 years
• In biological systems PB hydrolysed in milliseconds thanks to enzymes
where is trypsin produced in humans?
The digestive enzyme trypsin in humans is produced in the pancreas as an inactive form -trypsinogen
(inactive so it does not self-digest pancreas)
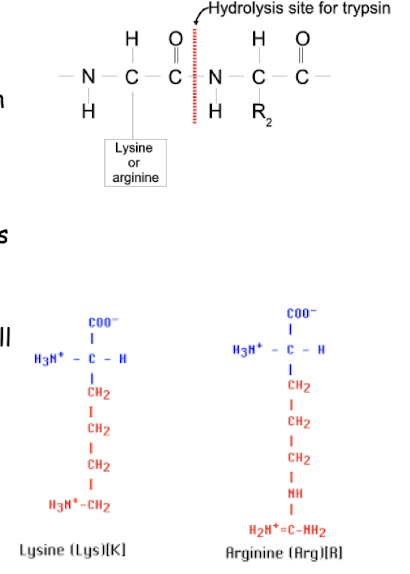
Trypsin catalyses the ________ of peptide bonds on the __________ of the amino acids _____________
If a proline residue is on the carboxyl side of the cleavage site, the cleavage will ________
hydrolysis , carboxyl side, arginine or lysine, cleavage will not occur.
how are proteases classified?
All organisms have proteases:
Classified into groups based on reactive amino acid in active sites i.e serine, cysteine, aspartate, threonine, glutamic acid proteases
Can also be classified based on the pH at which they are most active
• Acid, neutral or alkaline proteases
what type of protease is trypsin?
Serine protease – Trypsin and chymotrypsin
How can we measure the enzyme activity of trypsin?
Follow the release of product or disappearance of substrate with time
i.e in this experiment

If there is a difference in the absorbance of the substrate and product, we can follow activity using a spectrophotometer
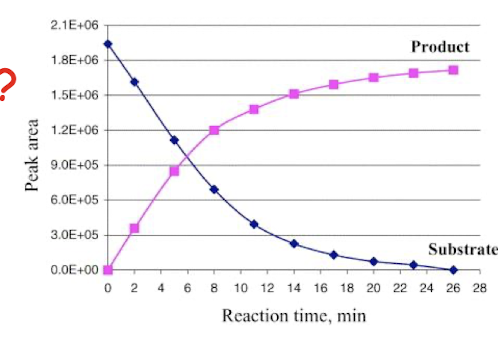
what is meant by a progress curve in relation to following enzyme activity?
If a fixed amount of enzyme is added to a fixed amount of substrate (usually in great excess), and the amount of product is determined at suitable intervals, we get a progress curve.
Is a curve because as the enzyme works the substrate conc. falls until eventually the rate of reaction is lowered. As the substrate runs out, the rate slows down to zero.
Monitoring the disappearance of substrate or the appearance of enzyme products is either ________________ or the reaction is ____________ and the amount of substrate/product is determined at ______________
continuous (i.e the reaction takes place within the detector) or stopped at intervals , fixed time points (the so-called fixed-time assay)
The early stage of the reaction (when the curve is at its steepest) is referred to as the ____________ and indicates the _______________
initial rate of enzyme activity, indicates the fastest rate that the enzyme can work under the current conditions
why is the Initial rate of enzyme activity a useful measurement & what is the SI unit of enzyme activity?
It can be converted to international units (IU) or SI units, to standardise expressions of enzyme activity:
The SI unit of enzyme activity, the katal, is defined as the amount of enzyme that catalyses the conversion of 1 mole of substrate into product in 1 second.
However this unit has never really caught on bc enzyme reactions are usually carried out in small volumes at very low concentrations (in the μmol range)
what is the ideal unit of choice for enzyme activity?
IU - international unit, defined as the amount of enzyme that will convert 1 μmol of substrate into products in 1 minute under defined pH and temperature conditions.
what segments of the earthworm were used in this experiment?
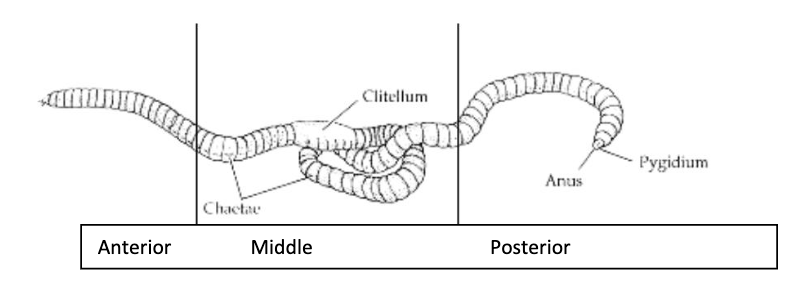
Each batch of worm segments was homogenized in approximately 4 volumes of phosphate buffered saline (PBS) and the homogenate centrifuged at 10,000 rpm for 20 mins.
The supernatant (i.e. the top fraction not containing any debris) was retained and labelled either A’ (anterior), ‘M’ (middle) or ‘P (posterior) depending on the sections of worm used
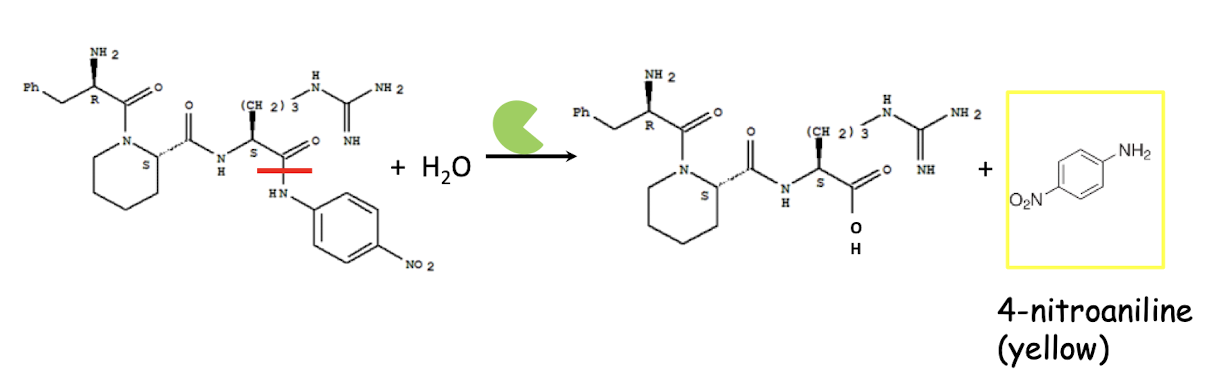
How do you determine trypsin activity in this experiment?
Fixed time (10 min) assay using a chromogenic substrate
• Chromozym TRY (substrate - artificial peptide)
• Carbobenzoxy-L-valyl-L-glycyl-L-arginine-4-nitranilide acetate
Trypsin cleaves peptide bond to release 4-nitroaniline (product)
Yellow appearance detected at 405-410 nm
what are the overall steps of determining the specific activity of trypsin in our earthworm extracts?
1) Determine the amount of product released from a chromogenic substrate by trypsin activity in the 3 earthworm extracts over 10 mins
2) Construct a protein standard curve (data provided) and use it to determine the amount of protein in 3 earthworm extracts
3) Determine the specific activity of trypsin in each extract
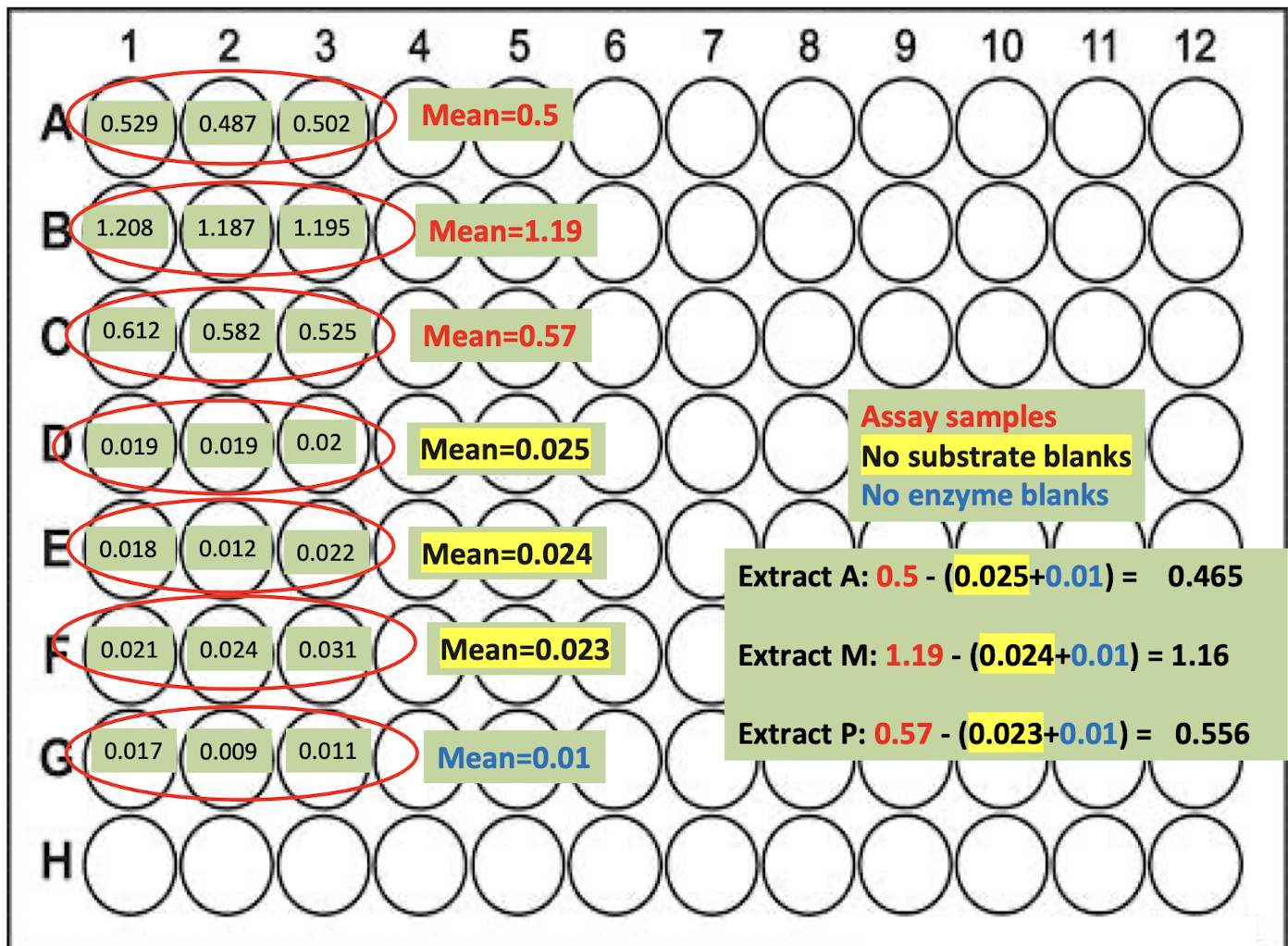
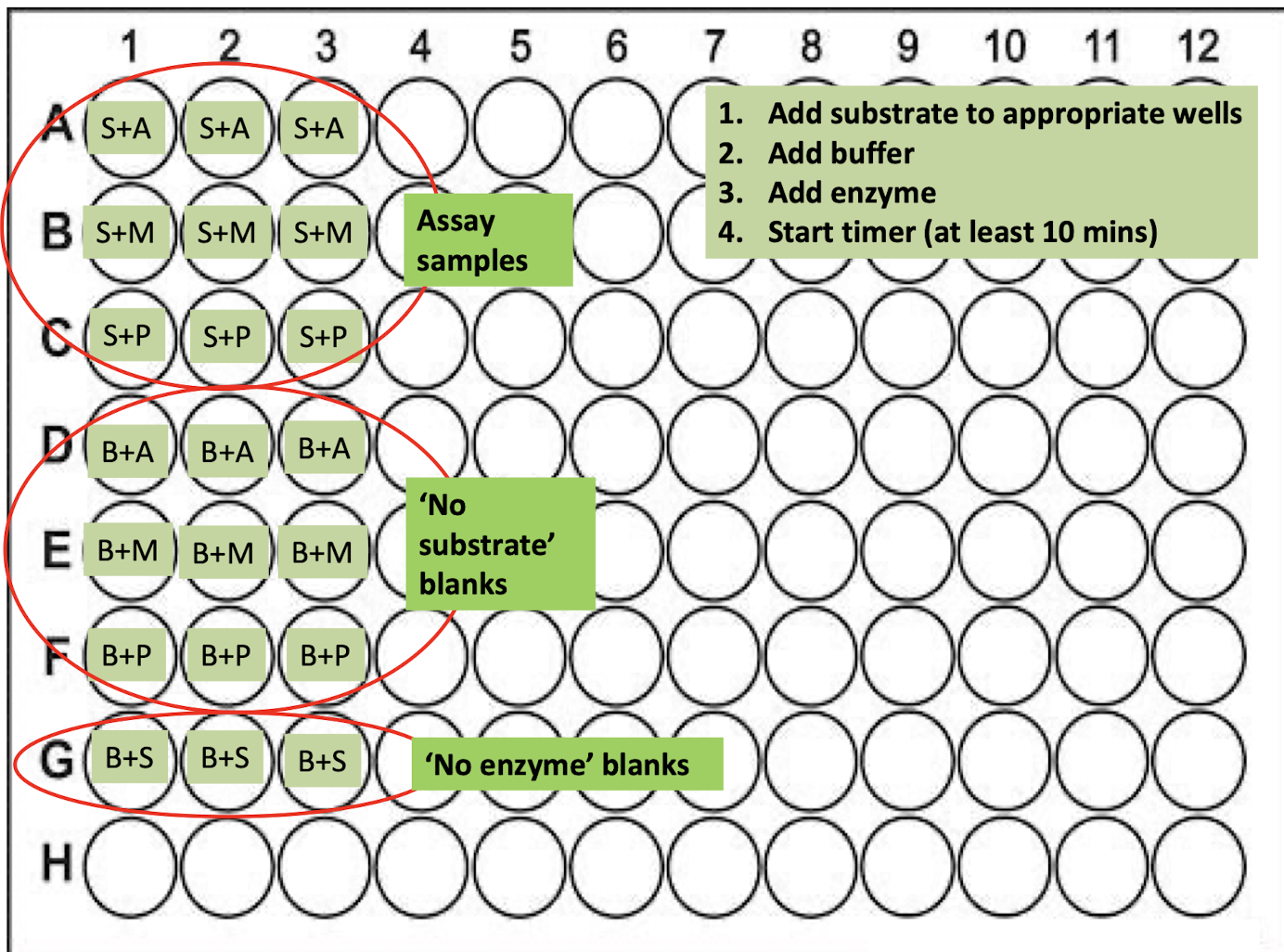
For each extract, how do we prepare the wells?
a) Ax3; Mx3; Px3 (9 in total) of experimental sample: 200 μl of substrate + 50 μl of each extract
b) Ax3; Mx3; Px3 (9 in total) of No substrate control: 200 μl of buffer + 50 μl of extract
c) x3 of No enzyme control: 200 μl of substrate + 50 μl of buffer
21 wells in total

what equipment are we provided with to conduct our enzymatic assay?
1. Microtitre plate
2. Substrate (chromozym-TRY)
3. TRIS Buffer
4. Earthworm extracts A, M and P

why is it important to start timing the assay once the enzyme is added?
Once you add the enzyme (earthworm extract) to the substrate
(chromozym-TRY) the reaction will start, so you should make sure you have all the wells prepared before you add the earthworm samples.
For each of the 3 earthworm extracts you need replicates of:
1. Experimental sample (Substrate + Earthworm extract)
2. No substrate control (Buffer + Earthworm extract)
3. No enzyme control (Substrate + Buffer)
The controls allow you to consider any absorbance that may arise from substrate or enzyme components.
Absorbance from these is subtracted from the experimental
samples to ensure that only the product arising from the action of the enzyme (trypsin) on the substrate (chromozym-TRY) is taken into account in the final calculation of enzyme activity:
Complete method:
1. Complete provided microtitre plate template to indicate what you will add to each well on the microtitre plate.
2. Pipette 200 μl Substrate to all the wells that should contain substrate i.e the exp samples & no enzyme samples
3. Pipette 50 μl of Buffer into 3 wells instead of earthworm extract (no enzyme controls)
4. Pipette 200 μl of Buffer into 9 wells instead of substrate (no substrate controls)
5. Pipette 50 μl of earthworm extract A into the appropriate wells
6. Pipette 50 μl of earthworm extract M into the appropriate wells
7. Pipette 50 μl of earthworm extract P into the appropriate wells
8. Leave the plate at room temperature for exactly 10 minutes, * 10 mins begins from the second you add the 1st earthworm extract. You may
need to incubate it for longer than 10 minutes, make note of the number of minutes you incubated it for.
9. Read absorbance of all wells on a microtitre plate reader at 405 nm. Use the phone camera to take a picture of the results on screen.
10. Use the Beer-Lambert Law (A=ɛcl) to determine the amount of product formed (in μmol).
what does an example of a complete microtiter plate template look like?
how do we calculate the specific enzyme activity in each earthworm extract?
By knowing the amount of protein present in each extract, this can be done using a microtiter BCA assay
what is a BCA (Bicinchoninic acid) assay?
The most widely used protocol in the ‘life sciences’.
Combines the well-known reduction of Cu2+ → Cu+ by protein in
an alkaline medium (the biuret reaction) with the highly sensitive and selective colourimetric detection of the cuprous cation (Cu+) using a unique reagent containing bicinchoninic acid.The purple-coloured reaction product of this assay is formed by the chelation of 2 molecules of BCA with one cuprous ion.
This water-soluble complex exhibits strong absorbance at 540 – 590 nm that is linear with increasing protein concentrations over a broad working range (20 – 2000 μg/ml), and therefore the Beer-Lambert law can be applied
how was the BCA assay carried out that gave the absorbance data given to us in the table?
•Added 50 μl of a protein standard (Bovine Serum Albumin) at different known concentrations to a well on a microtitre plate
• Added 50 μl of each extract to 9 separate wells
• Added 200 μl of BCA reagent and incubated at 37ºC for 30 min
• Read absorbance 550nm
• Table in manual – absorbance readings from BCA assay