organic chemistry 1: nomenclature
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
38 Terms
![<p><span>This is a/an </span><span style="color: mediumseagreen"><strong>[... group]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/e26997dc-7604-4422-895c-16282f01c2ca.png)
This is a/an [... group]
formyl group
![<p><span>This is </span><span style="color: mediumseagreen"><strong>[name]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/70df976c-06e3-4b37-9f8d-3b66bf2ebd89.png)
This is [name]
formaldehyde
![<p><span>This is </span><span style="color: mediumseagreen"><strong>[name]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/0358e570-1b01-41ae-810b-7eecb8b1d948.png)
This is [name]
formic acid
![<p><span>This is a/an </span><span style="color: mediumseagreen"><strong>[... group]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/bdcbb3e5-d447-469c-9a46-77df3c3c3119.png)
This is a/an [... group]
acetyl group
![<p>This is <span style="color: mediumseagreen"><strong>[name]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/c9d330e9-7281-484b-be3b-22de03c8e7cd.png)
This is [name]
acetaldehyde
![<p>This is <span style="color: mediumseagreen"><strong>[name]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/095b433a-5362-4b9f-b98a-e72c7eb00783.png)
This is [name]
acetic acid
![<p>This is <span style="color: mediumseagreen"><strong>[name]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/d6381f70-f858-4b6a-906d-49459eddfed0.png)
This is [name]
acetone
![<p>This is <span style="color: mediumseagreen"><strong>[name]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/bd5a472e-a504-4fd7-a70b-0c670b349188.png)
This is [name]
acetylacetone
![<p>This is <span style="color: mediumseagreen"><strong>[name]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/4e91c230-7d82-478e-85fa-d2b57d380e48.png)
This is [name]
acetophenone
![<p><span>This is a/an </span><span style="color: mediumseagreen"><strong>[... group]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/a02ac38f-b362-4188-88f9-401c052ec49a.png)
This is a/an [... group]
benzyl group
![<p><span>This is </span><span style="color: mediumseagreen"><strong>[name]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/d2af228c-57c6-4663-9b85-c1860f9521f5.png)
This is [name]
benzaldehyde
![<p><span>This is </span><span style="color: mediumseagreen"><strong>[name]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/86caba27-1441-4ab4-9a2f-a113284d1c60.png)
This is [name]
benzoic acid
![<p><span>This is </span><span style="color: mediumseagreen"><strong>[name]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/0e285be7-fc8f-4d71-8059-7236f4ff36f3.png)
This is [name]
benzoin
![<p><span>This is </span><span style="color: mediumseagreen"><strong>[name]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/29886125-5109-4799-9f32-70cb4b97efbf.png)
This is [name]
styrene
![<p><span>This is a/an </span><span style="color: mediumseagreen"><strong>[... group]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/dd6274c9-bc36-4888-a9f0-a20118688952.png)
This is a/an [... group]
acryl group
![<p><span>This is a/an </span><span style="color: mediumseagreen"><strong>[... name]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/e20cb536-7e80-4485-a6c8-d33d88cb1405.png)
This is a/an [... name]
acrolein
![<p><span>This is a/an </span><span style="color: mediumseagreen"><strong>[... name]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/e526cc7b-ca7c-4ba0-8767-fb2d741a6134.png)
This is a/an [... name]
acrylic acid
![<p><span>This is a/an </span><span style="color: mediumseagreen"><strong>[... group]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/ef970c6d-fd97-4379-b690-646dadbedba9.png)
This is a/an [... group]
carboxyl group
![<p><span>This is a/an </span><span style="color: mediumseagreen"><strong>[... ion]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/d06facca-6c72-4627-b7fd-dd3b4071a37d.png)
This is a/an [... ion]
carboxylate ion
![<p><span>This is a/an </span><span style="color: mediumseagreen"><strong>[... ion]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/08475085-f787-410d-9ecb-dc7ae32bc998.png)
This is a/an [... ion]
carbonate ion
![<p><span>This is </span><span style="color: mediumseagreen"><strong>[name]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/6fc0539b-c91a-45b2-bab7-8c362ce06e57.png)
This is [name]
carbonic acid
[...] are hydrocarbons in which all carbon–carbon bonds are single bonds
Alkanes
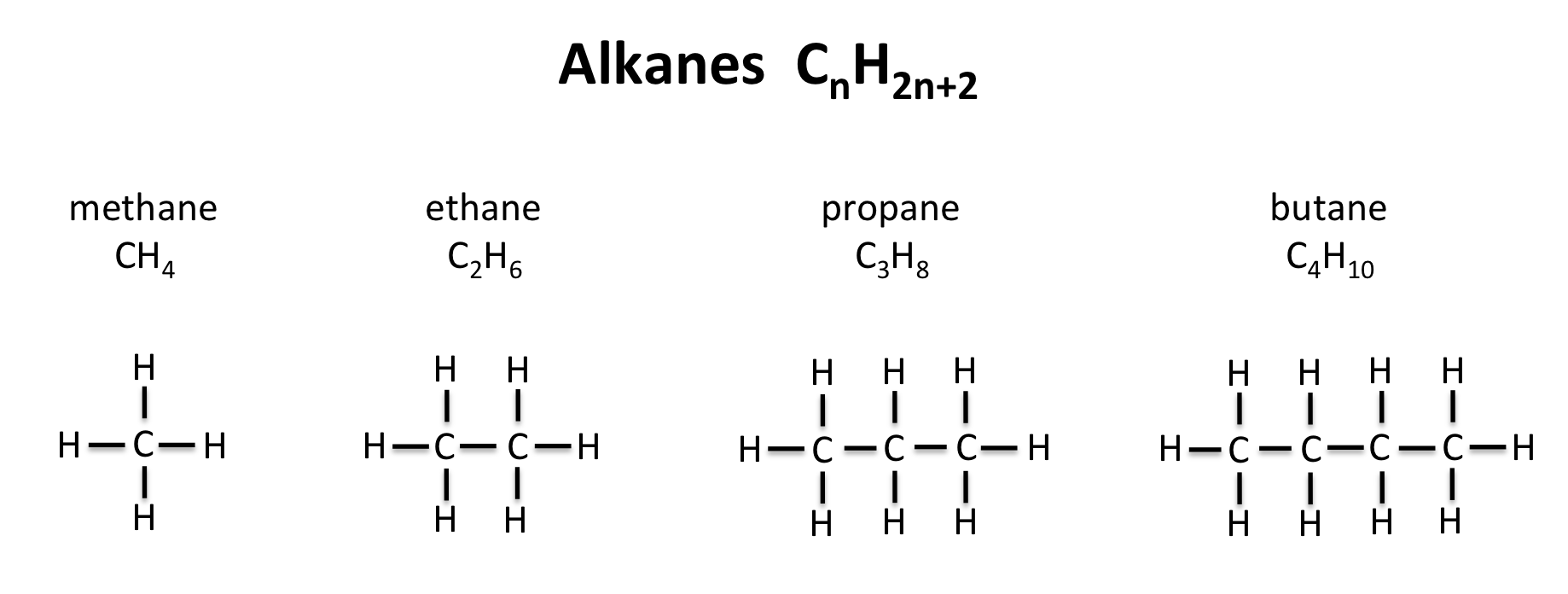
Alkanes are named according to the number of [...] present followed by the suffix [...]
carbon , -ane
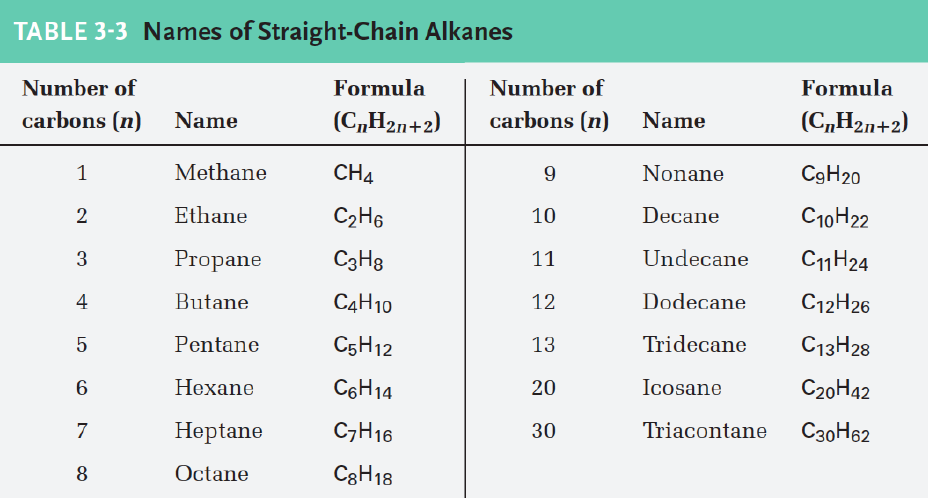
Alkenes have a [...] bond and use the suffix [...]
double, -ene
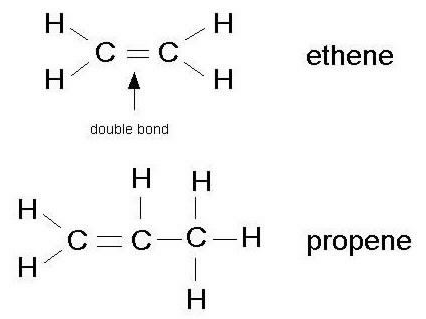
Alkynes have a [...] bond and use the suffix [...]
triple, -yne
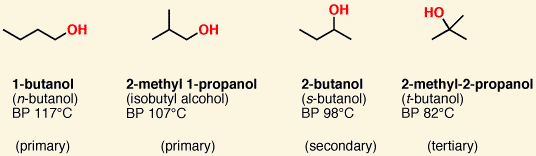
Alcohols have a/an [...] group and use the suffix [...] or prefix [...]
-OH, -ol, hydroxy
alcohols have higher priority than double or triple bonds
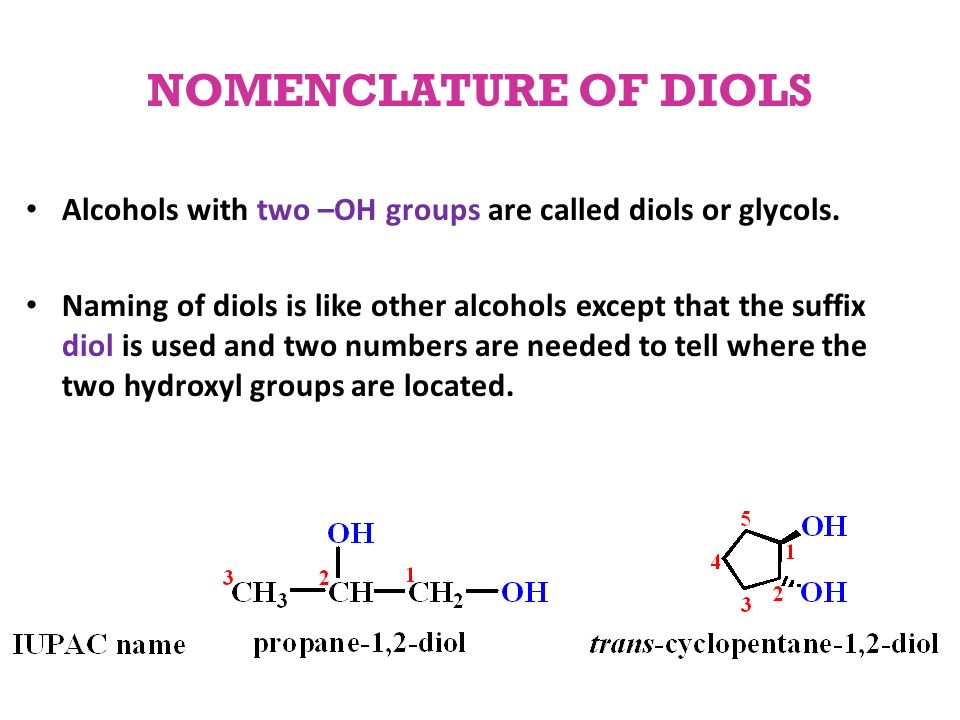
Diols contain two [...] groups
hydroxyl (OH)
![<p><span>This compound is named </span><span style="color: mediumseagreen"><strong>[...]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/49566396-9f5f-4cdc-89c2-0b887e8535e4.png)
This compound is named [...]
methane
![<p><span>This compound is named </span><span style="color: mediumseagreen"><strong>[...]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/594a55a9-4348-484d-a66f-973ac1833726.png)
This compound is named [...]
propane
![<p><span>This compound is named </span><span style="color: mediumseagreen"><strong>[...]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/c201f81c-ef6a-48f8-a074-79806e149116.png)
This compound is named [...]
butane
![<p><span>This compound is named </span><span style="color: mediumseagreen"><strong>[...]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/325adf44-ceed-4946-9b1e-149c413ed58d.png)
This compound is named [...]
pentane
![<p><span>This compound is named </span><span style="color: mediumseagreen"><strong>[...]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/6bcf3b41-553a-4345-9773-34d927dd56ed.png)
This compound is named [...]
hexane
![<p><span>This functional group is called a/an </span><span style="color: mediumseagreen"><strong>[...]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/298e6b0c-69b2-4eb1-9c0f-3449e8d5e36f.png)
This functional group is called a/an [...]
carbonyl group
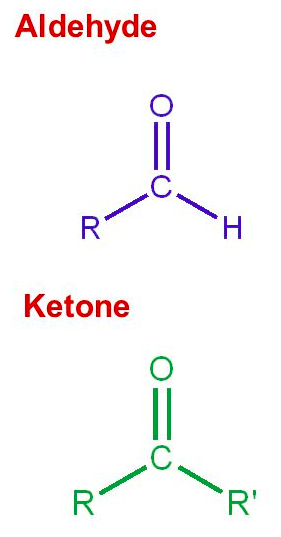
Aldehydes have a carbonyl group on the [terminal or non-terminal] carbon
terminal carbon
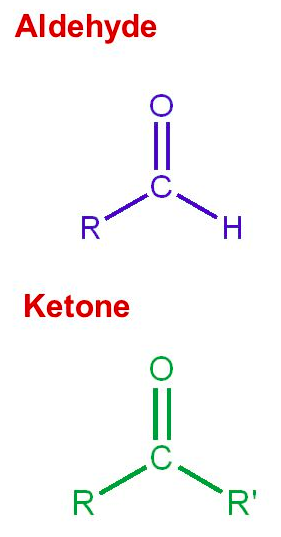
Ketones have carbonyl group on a [terminal or non-terminal] carbon
non-terminal carbon
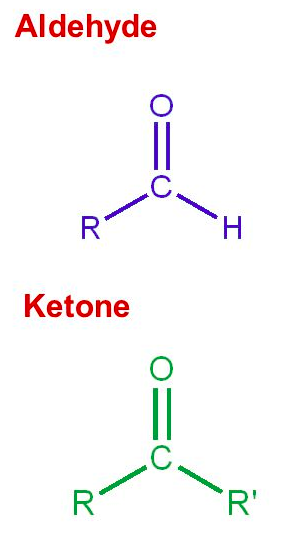
Alcohols are classified as 1º, 2º, and 3º alcohols according to the number of carbons attached to the [...]
carbon bearing the hydroxyl group
in other words, whether the hydroxyl is bound to a primary, secondary or tertiary carbon.
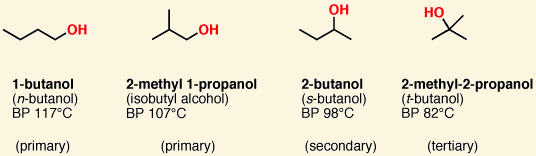
Amines are classified as 1º, 2º, and 3º amines according to the number of carbons attached to the [...]
nitrogen atom
[...] is the highest priority functional group
carboxylic acid
this is because it contains 3 bonds to oxygen