IB Chemistry Unit 14 - Chemical Equilibrium
1/41
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
42 Terms
Equilibrium
When the rates of the forward and reverse reactions are equal.
Chemical Equilibrium
The identity of the substance changes, and sometimes the state does as well.
Chemical Equilibrium Example
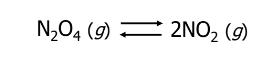
Physical Equilibrium
ONLY the state of the substance changes.
Physical Equilibrium Example
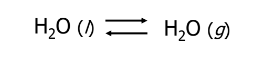
Mass Action Expression (MAE) is also known as…
The Equilibrium Constant / The Kc
The Mass Action Expression (MAE) shows…
the relationship between the concentrations of the reactants and products in a chemical equilibrium.
Kc = Kp ONLY when…
Δn = 0
Kp Equation
Kp = Kc(RT)Δn
A Small Kc indicates…
That the reaction favors the reactants.
A Large Kc indicates…
That the reaction favors the products.
A homogeneous equilibrium involves a reaction where all substances are in _____ phase.
the same
A heterogeneous equilibrium involves a reaction where all substances are in ________ phases.
different
When finding Kc, we express the concentrations of Aqueous Solutions and Gases, but NOT…
Pure Solids & Pure Liquids
If the reaction is multiplied by [a factor], the Original Kc is…
raised to the power of [the factor].
When looking at the reverse reaction, you find the _____ of the Kc.
reciprocal (1/Kc)
If there are multiple reactions with Kcs, the net reaction Kc is found by…
Multiplying all the Kcs.
Concentrations in MAE Units
mol/L
Gases in MAE Units
atm
____ and ____ are considered unitless quantities.
Kp and Kc
Stoichiometric coefficients affect…
the MAE.
When you don’t know whether or not the reaction is at equilibrium, you find the…
Qc
Qc < Kc
The reaction needs to proceed towards products.
Qc > Kc
The reaction needs to proceed towards reactants.
Qc = Kc
The reaction is at equilibrium.
Le Châtelier’s Principle
If an external stress is applied to a system at equilibrium, the system will adjust to offset the stress and reach a new equilibrium.
Factors that AFFECT Equilibrium
Concentration, Volume & Pressure, Temperature, Catalysts
Factors that SHIFT Equilibrium
Concentration, Volume & Pressure, Temperature
Concentration of Products INCREASES
Shift Left ←
Concentration of Products DECREASES
Shift Right →
Concentration of Reactants DECREASES
Shift Left ←
Concentration of Reactants INCREASES
Shift Right →
Pressure INCREASES
Shift to the side with LEAST moles
Pressure DECREASES
Shift to the side with MOST moles
Volume INCREASES
Shift to the side with MOST moles
Volume DECREASES
Shift to the side with LEAST moles
Does a Catalyst shift Equilibrium?
No
Catalysts lower the…
Ea
Temperature INCREASES in an Exothermic Reaction
Shift Left ←
Temperature DECREASES in an Exothermic Reaction
Shift Right →
Temperature INCREASES in an Endothermic Reaction
Shift Right →
Temperature DECREASES in an Endothermic Reaction
Shift Left ←