unit 3: quantities in chemical reactions
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
accuracy
How close a given quantity is to the accepted value.
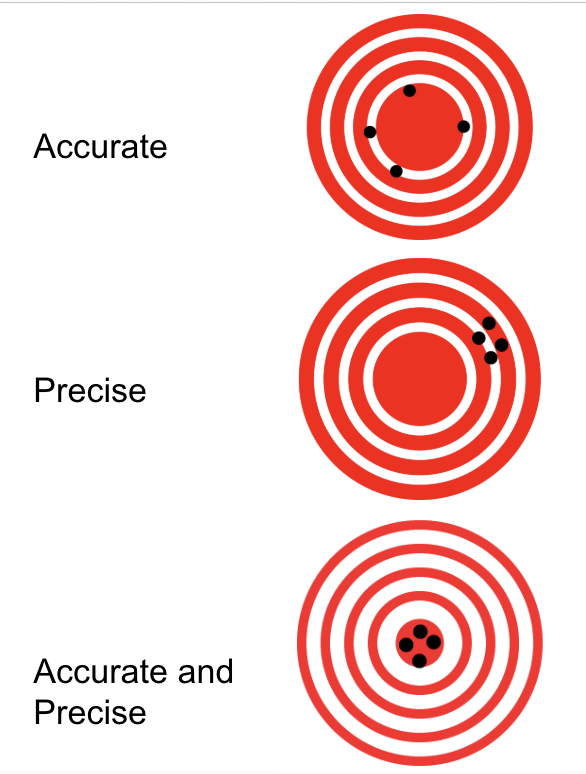
Precision
How exact a measurement is. How close measurements are to each other.

sig figs
The number of digits used to express a number to the required degree of precision
sig fig rules
All Non zero numbers are significant |
All zeros located between non zeros are significant. |
Zeros to the left of non zeros are not significant. |
Zeros to the right of non zeros are significant only when decimal present |
Exact numbers, for example the number of meters in a kilometer or counted numbers have infinite sig figs. |
scientific notation and sig figs
Is used to express very large or very small numbers.
E.g 10 492 000 m
1.0492 x 107 m
Can use scientific notation to round to the correct number of sig figs:
3 498 to 2 sig figs = 3.5 x 103
multiplying/dividing sig figs
Multiplying and Dividing
Your answer should have the same total digits as the number in the question with the fewest total significant figures.
Ex. 2.34 cm x 1.5 cm
= 3.5cm²
2. Adding and Subtracting
Express your answer with the smallest number of Decimal Places used in the question.
Ex. 4.35 kg + 0.346kg
= 4.70 kg
3. Rounding
f the number after your last significant figure is more than five then round up one number.
Ex. 2. 346 to three sig figs = 2.35
b) If the number after your last significant figure is less than five then leave the number as is.
Ex. 5.73 to two sig figs = 5.7
Rounding pt 2
If the number ends in five after the last significant figure (with nothing after the 5), this is done two ways.
If the last significant figure is odd, round up.
Ex. 18.35 (3 sig figs) = 18.4
If the last significant figure is even,leave it as is.
Ex. 18.25 (3 sig figs) = 18.2
atomic masses
What do the atomic masses on the periodic table represent?
E.g. C has an atomic mass of 12.01 amu (atomic mass units).
What is the actual mass of one C atom? 1.99 × 10^-23
molecules vs formula units
Covalent compounds (e.g. H2O) are composed of molecules
Ionic compounds (e.g. NaCl) are composed of formula units
the mole
Contains 6.02 x 1023 particles
n = moles (mol)
N = number of particles
NA= Avogadro's number
(6.02 x 1023 particles/mol)
n = N/NA
molar mass
Mass of 1 mole of substance (6.02x1023 particles)
Units: g/mol
mass to moles
n = moles (mol)
m = mass (g)
M = molar mass (g/mol)
n = m/M
percent composition
for actual masses:
%X = mx/mtotal x 100
for molar masses:
%X = Mx / Mtotal x 100
hydrates
to find percent of water:
find mass of just water
use mass of just water, hydrate, to find % of water
to find the formula of the hydrate:
find moles of water
find moles of anhydrous
x = mol H2O / mol anhydrous
hydrates definition
An ionic compound that has a specific number of water molecules bound to each formula unit.
Solids only - water molecules released when aqueous
anhydrous
An ionic compound that has no attached water molecules.
Eg. ZnSO4
empirical formula
The lowest whole number ratio of atoms, for example: CH2O
molecular formula
The actual number of atoms in a compound, for example:
CH2O or C6H12C6
to get empirical formula
% to mass: Assume 100g total
Mass to moles: Convert to moles
Divide by the smallest: Divide each value by the smallest number of moles to find the ratio
Multiply until whole: multiply the numbers in the ratio to get whole number subscripts
to get molecular formula
Find the molar mass of the empirical formula
Divide actual molar mass (provided in the question) by empirical molar mass to get multiplication factor X
Multiply the subscripts in the empirical formula by this multiplication factor (X, the answer from step two )
balanced chemical equations for stoichiometry
4 NH3(aq) + 5O2(g) → 6H2O (l) + 4NO(g)
The balanced chemical equation has the same number of each type of atom on each side (The Law of Conservation of Mass)
For every 4 mol NH3 used, we need 5 mol O2, which produces 6 mol H2O and 4 mol NO
This ratio does NOT work for direct conversion from mass to mass because each compound has a different molar mass
mole ratio
These problems require a balanced chemical equation.
The fraction that we use to determine the new number of moles is called a mole ratio.
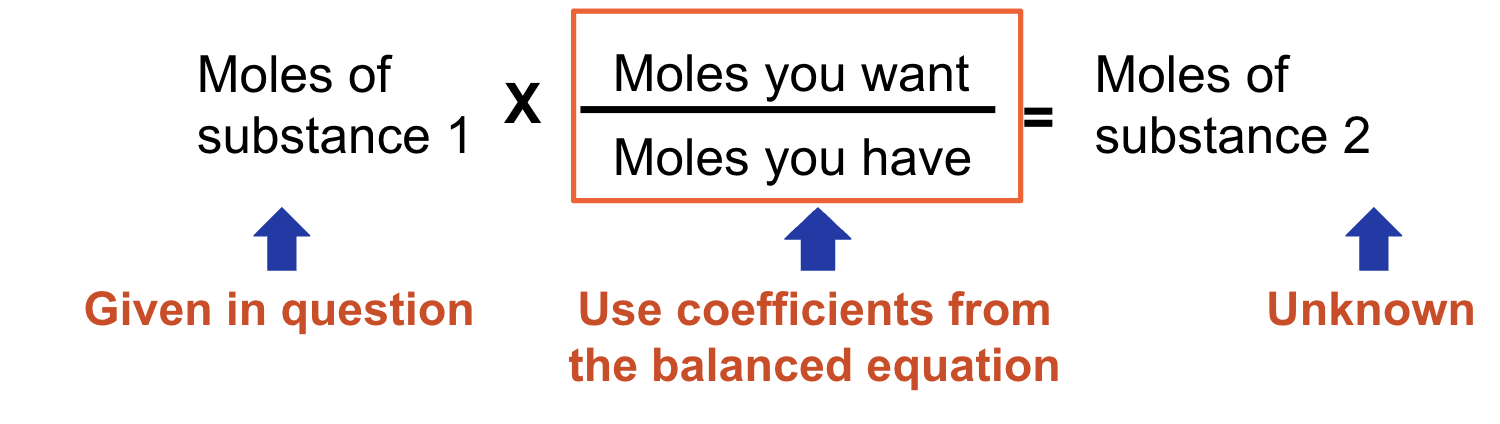
stoich mass to mass
Balance equation
Convert to moles
Mole ratio
Convert from moles to desired quantity (mass, particles etc)
percent yield
Many reactions do not go to completion
Not all reactants will be converted to products
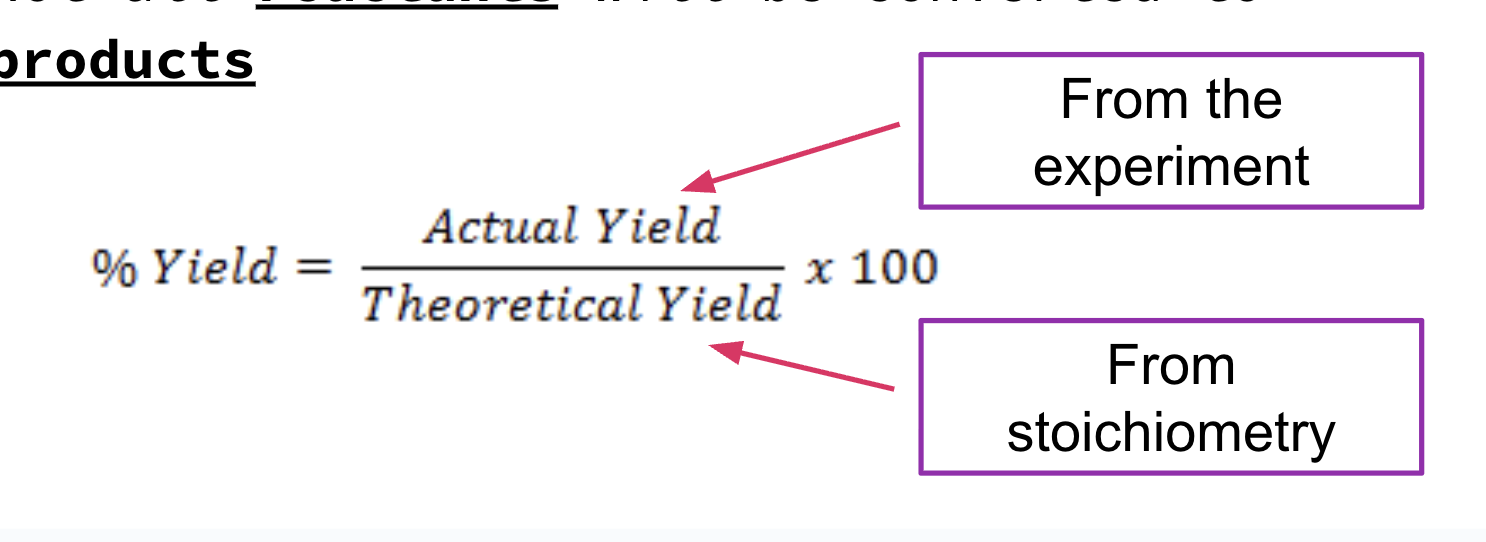
sources of error
Not all product recovered
Not all the product makes it onto the scale to be weighed (NOT due to a spill but something that happens even if the procedure is followed perfectly)
Equipment limitations
Even if functioning perfectly, all devices have limits in accuracy (a pipette measurement is accurate to 2 decimal places, a graduated cylinder to 1 decimal place)
Reactant impurities
If a reactant purchased from the manufacturer is 95% pure (higher purity is more expensive), less starting material is available to react, and less product will be made (your calculations are based on 100% purity).
Side reactions occur
One or more of the starting materials react to create a different product. Therefore, less reactant is available to make the desired product. The unwanted product could be lighter or heavier than the desired product.
Product is not dry
Water is also being weighed or a hydrate has formed making the product appear heavier
limiting reagent
The reactant that limits the reaction and produces the least amount of product
excess reagent
There will be some of the other reactant left over and it is called the “excess reagent”