Molecular Bio Final Exam
1/97
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
98 Terms
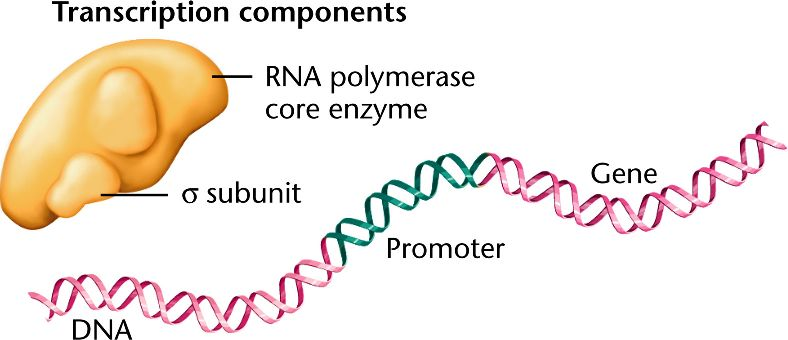
Sigma factors
subunits of RNA polymerase (RNAP) that help it recognize and bind to promoter regions by forming weak bonds with specific DNA sequences.
DNA Binding Specificity
-35 region: Bound using a helix-turn-helix motif.
-10 region: Binding is more complex and variable.
Diversity & Roles
Act as “master regulators” of gene expression in response to environmental changes.
Examples of Sigma Factors:
σ⁷⁰: Active during exponential growth.
σᴴ: Activated in response to heat shock.
σᴱ: Responds to envelope stress.
Bacillus species: Have ~18 sigma factors, reflecting complex regulation.
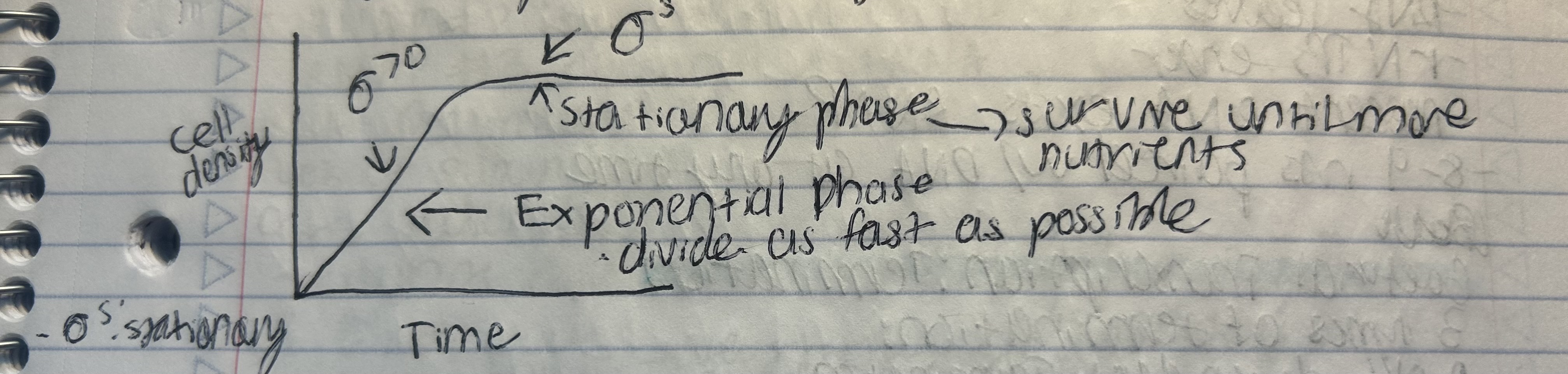
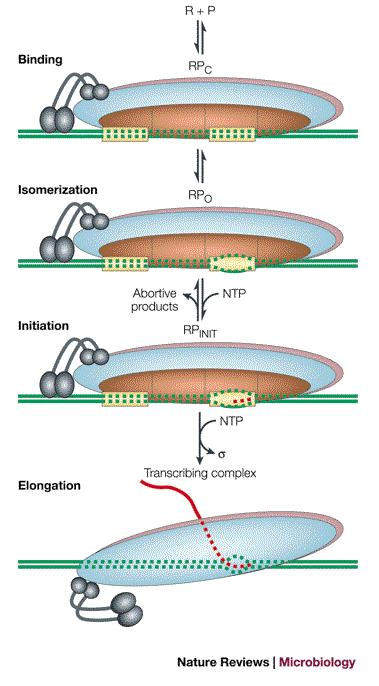
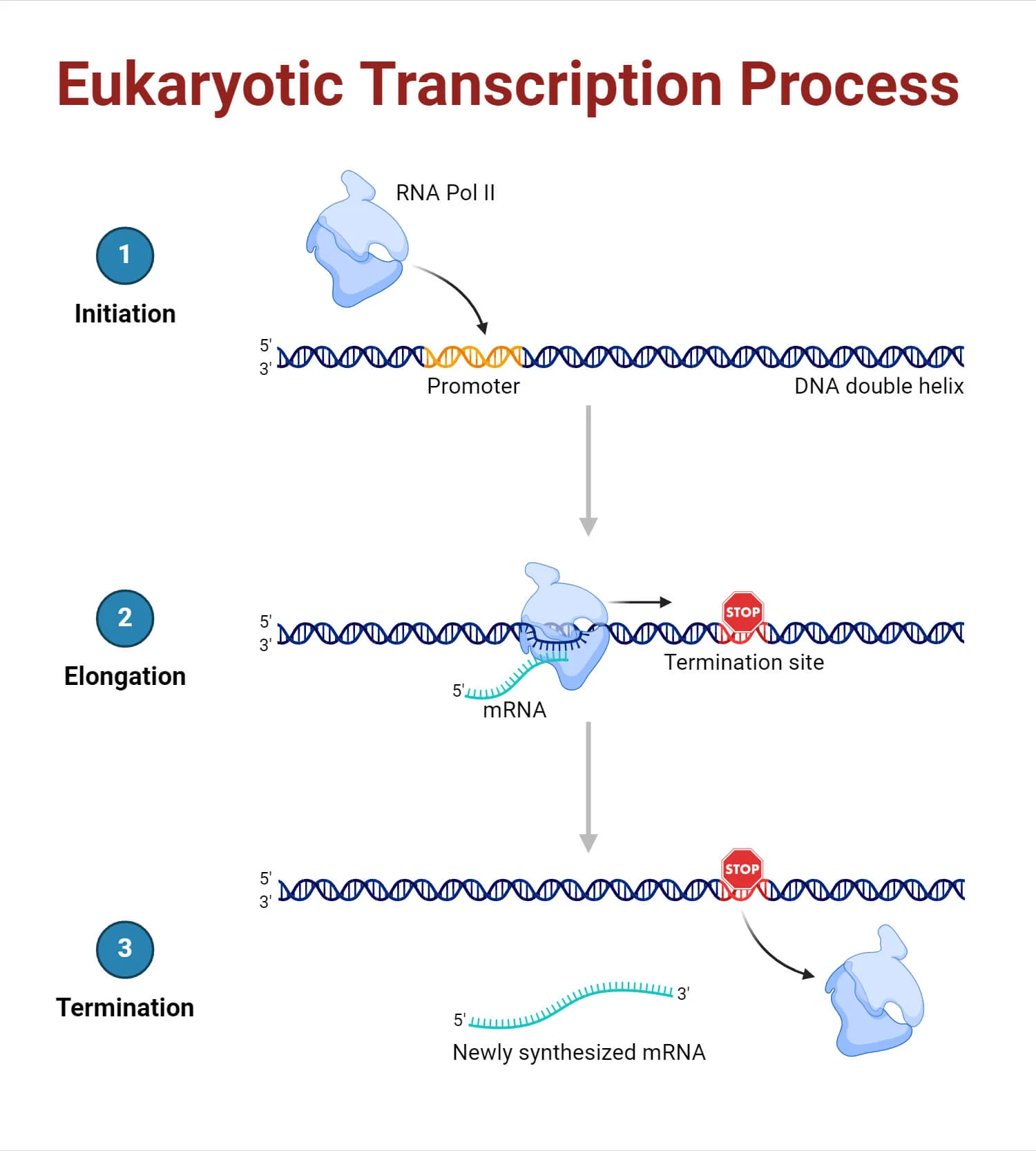
Bacterial Transcription: Initiation
DNA Melting (Irreversible Step):
RNAP separates the DNA strands at the promoter to create an open complex.Abortive Initiation:
RNAP repeatedly starts transcription but releases short RNA fragments (up to ~9 nucleotides) before fully escaping the promoter.Promoter Escape:
Weak interactions initially hold RNAP at the promoter.
RNAP builds up tension by pulling in DNA (called "scrunching"), which stores energy.Scrunching Model:
Explains how the energy from DNA scrunching helps RNAP break free from the promoter and transition to elongation.
Bacterial transcription: elongation
Sigma Factor Release:
Sigma factor stays behind at the promoter.
Core RNAP continues synthesizing RNA on its own.
RNA-DNA Pairing During Elongation:
DNA enters the RNAP active site.
Incoming rNTPs (RNA nucleotides) are added to the growing RNA chain.
Nascent (newly made) RNA exits the RNAP.
Typically, 8–9 nucleotides of RNA remain base-paired with the DNA at any time.
Bacterial transcription: termination
Termination is the process of ending transcription — when RNA polymerase (RNAP) stops and detaches from DNA.
3 Main Types of Termination:
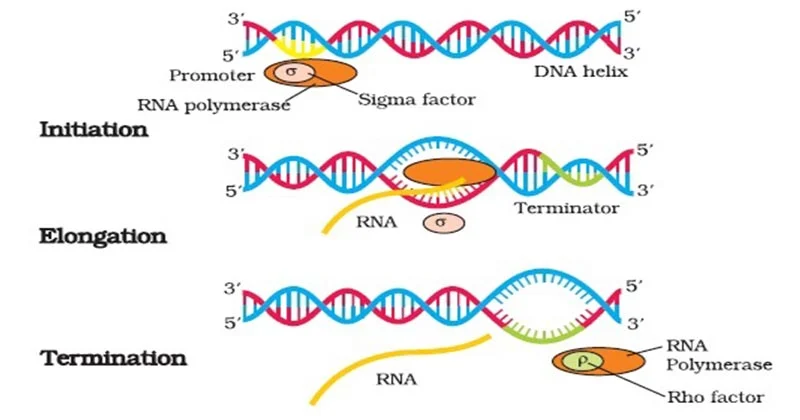
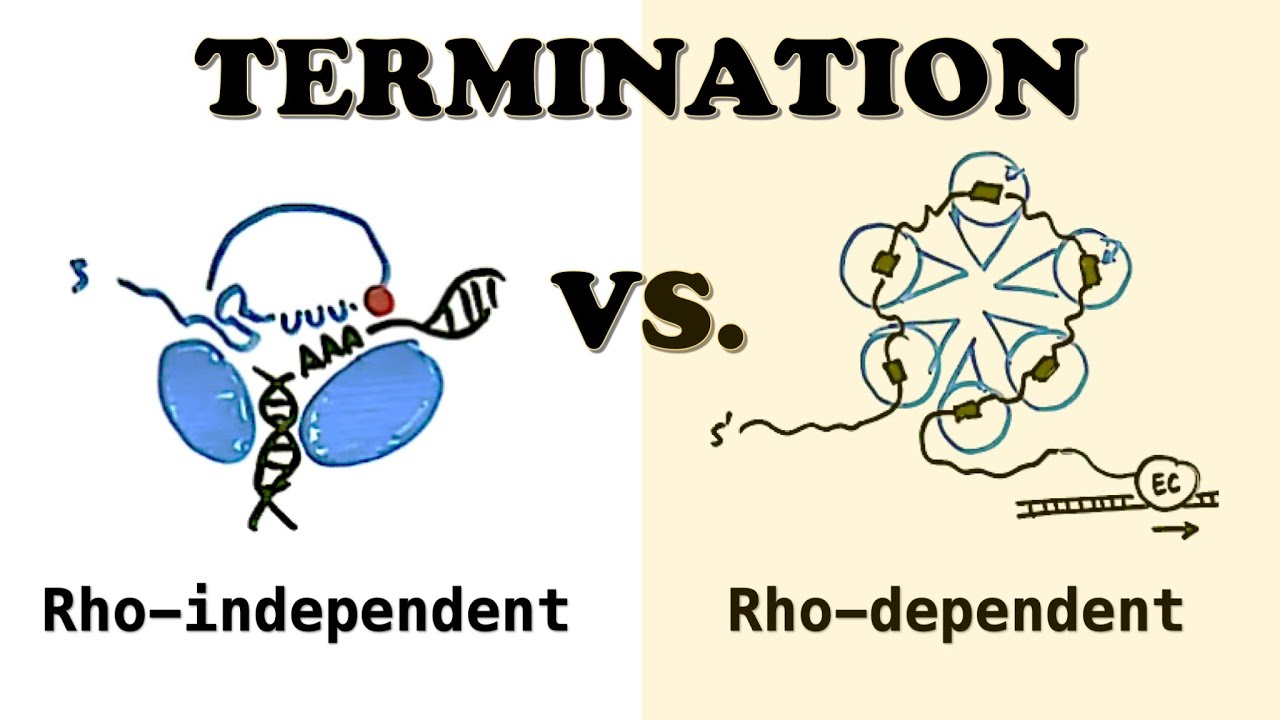
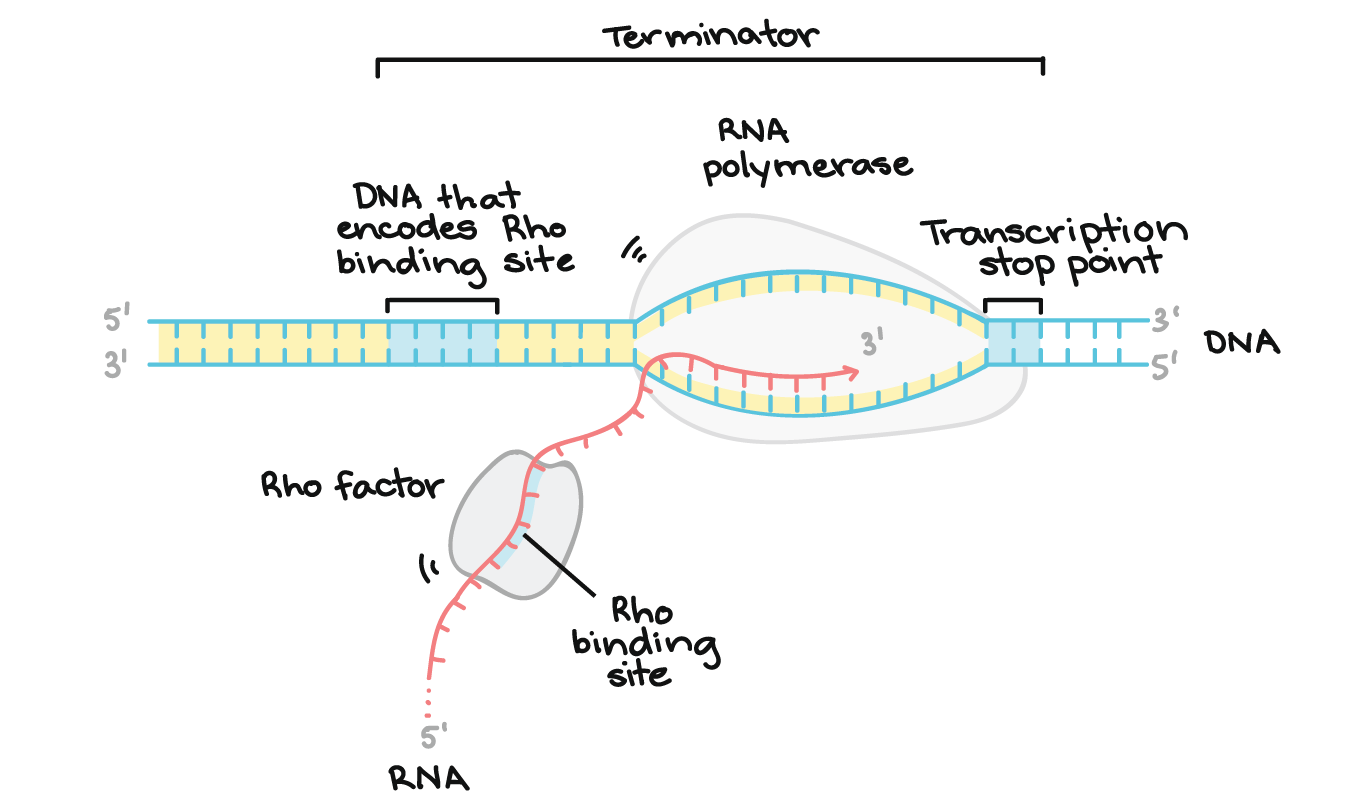
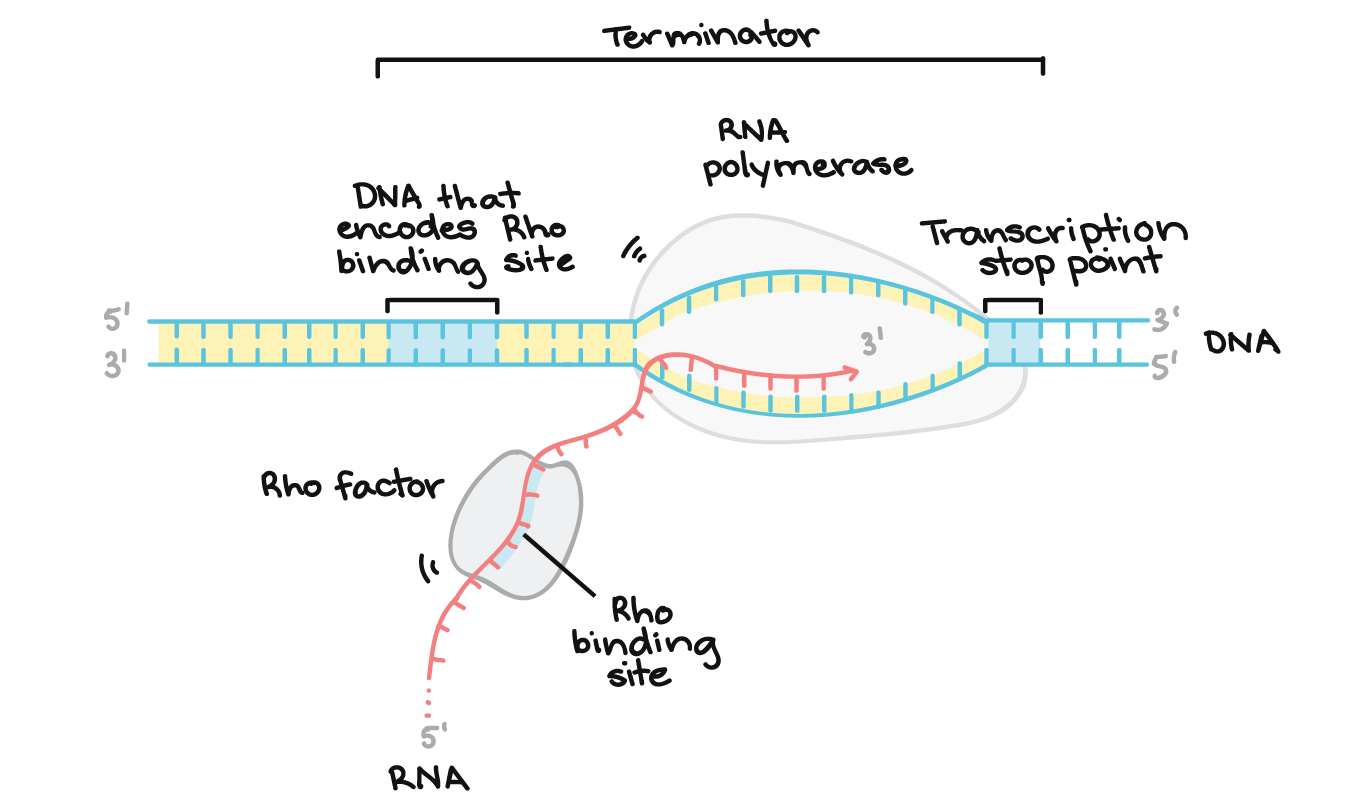
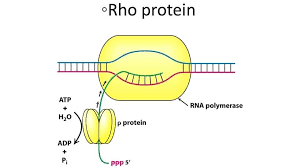
1) Rho-Dependent Termination
Involves Rho, a ring-shaped protein.
Rho binds the RNA and uses ATP to travel along it.
When Rho catches up to RNAP, it causes RNAP to fall off the DNA.
2) Rho-Independent Termination (Intrinsic Termination)
RNA forms a GC-rich hairpin followed by a string of U’s (poly-U tail).
This structure disrupts RNAP, causing it to detach.
Happens in about 50% of bacterial transcripts.
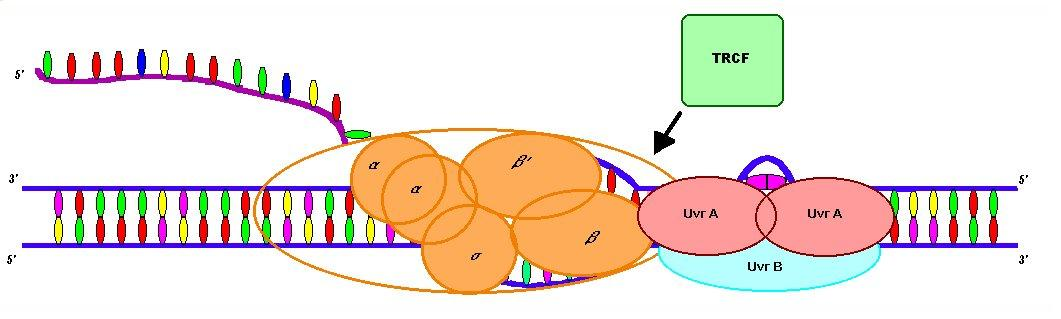
3) TRCF-Mediated Termination (Transcription Repair Coupling Factor)
TRCF is another ATP-powered ring-like protein.
It moves along DNA, searching for stalled RNAP.
If it finds one, it knocks it off, ending transcription.
Key Reminder:
Transcription officially ends when RNAP leaves the DNA template.
Transcription in Eukaryotes
Eukaryotic RNA Polymerases
RNA Polymerase I (RNAP I): makes most rRNA.
RNA Polymerase II (RNAP II): makes most mRNA.
RNA Polymerase III (RNAP III): makes tRNA and some rRNA.
Structure of RNA Polymerase II
Composed of multiple subunits:
Core subunits include versions similar to bacterial β, β', 2×α, and ω.
Common subunits: shared across RNAP I, II, and III. Their exact functions are mostly unknown (mutants are lethal, so hard to study).
Non-essential subunits: can be deleted without killing the cell, but the cells become very sick (impaired function).
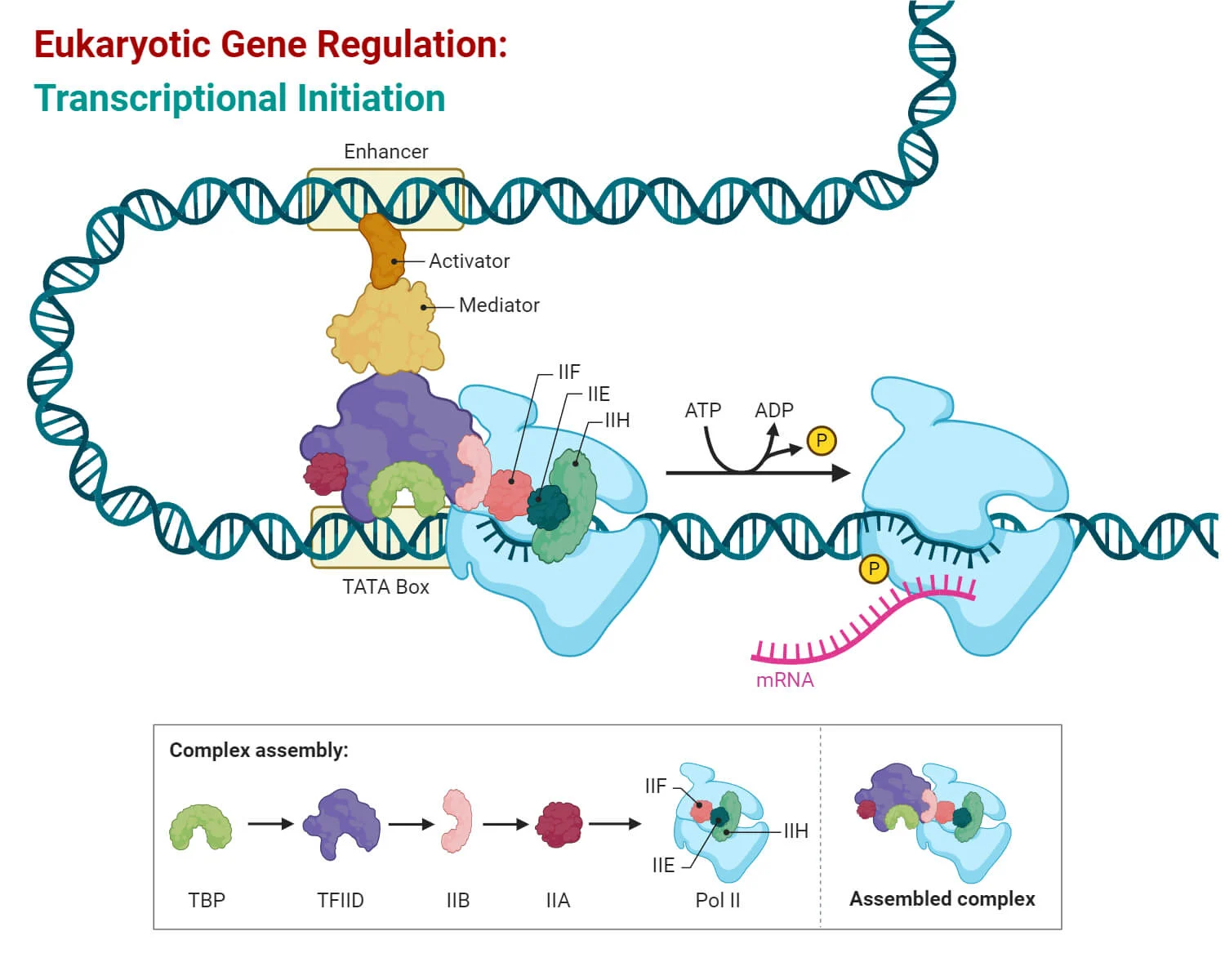
Eukaryotic transcription: initiation-promoters
Class II promoters are DNA sequences that direct RNA polymerase II (RNAP II) to start transcription of protein-coding genes.
Key Points:
These promoters were identified by comparing many gene sequences to find shared patterns called consensus sequences (like the TATA box).
Transcription factors bind to these promoters and change the shape (topology) of the DNA to help RNAP II bind and start transcription.
Why It Matters:
These promoters are essential for properly turning on genes that make mRNA → proteins.
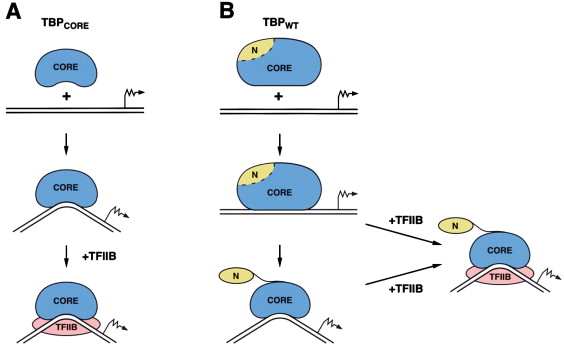
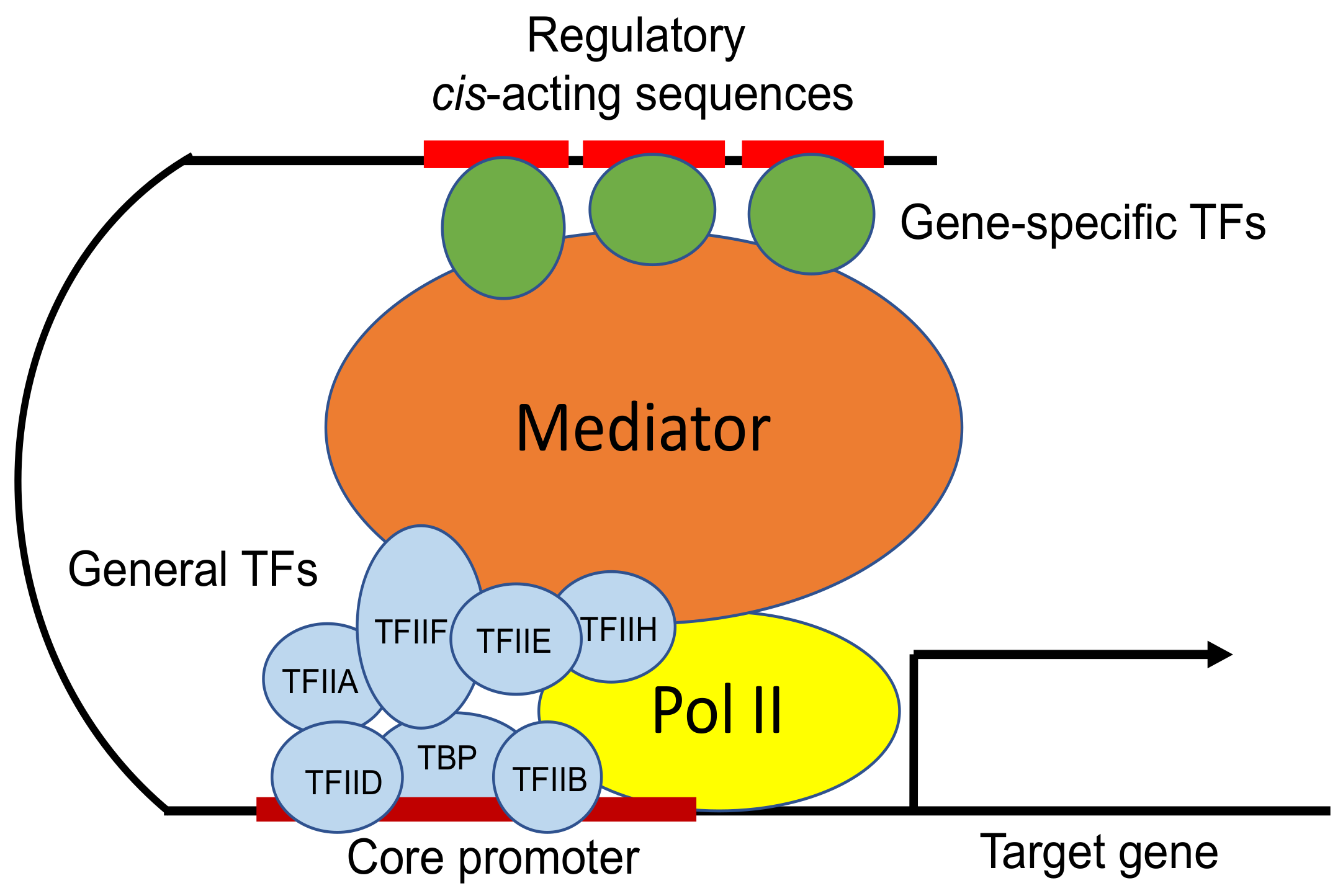
TBP-DNA complex
A critical part of the transcription initiation process in eukaryotes, involving TBP and the mediator complex.
Key Components:
TBP (TATA-Binding Protein):
Binds to the TATA box in the promoter region
Bends the DNA to help position the rest of the transcription machinery
Mediator Complex:
A large protein complex that connects transcription factors (TFs) to RNA polymerase II
Can interact with TFs hundreds to thousands of nucleotides away from the gene
Regulates transcription by helping coordinate signals from enhancers and activators
Control of eukaryotic transcription
RNA Polymerase II Carboxy Terminal Domain (CTD)
Acts as a “communication center” between RNAP II and proteins involved in transcription (e.g., transcription factors, capping enzymes, elongation factors).
Unmodified CTD:
Allows RNAP II to bind transcription factors (TFs) and the promoter for transcription initiation.
Phosphorylated CTD (at Serine 5):
Triggers initiation and promoter escape.
Begins recruitment of RNA processing factors (like 5′ capping enzymes).
Eukaryotic Transcription: elongation
Elongation Factors
After initiation, rapid changes occur:
CTD phosphorylation pattern changes — Serine 2 is phosphorylated.
This allows elongation factors to bind.
The 5′ end of the RNA is capped early in elongation
CTD (C-Terminal Domain) of RNAP II
Composed of heptapeptide repeats (7 amino acids per repeat).
The sequence and number of repeats vary across species.
Phosphorylation of CTD Serines
Primarily involves serines (especially Ser5 and Ser2).
Phosphorylation Patterns:
Unphosphorylated CTD: Binds to the promoter (initiation stage).
Serine 5 phosphorylation: Triggers promoter escape.
Serine 2 phosphorylation: Signals the start of elongation.
Eukaryotic transcription: elongation (RNA capping)
RNA capping is the process of adding a 7-methylguanosine cap to the 5′ end of a newly made RNA using a unique triphosphate bridge.
When Does It Happen?
Happens very early — after just ~30 nucleotides of RNA are made.
Functions of the 5′ Cap:
Protects the RNA from degradation by RNases
Acts as a signal for RNA export from the nucleus
Helps the ribosome recognize the RNA for translation
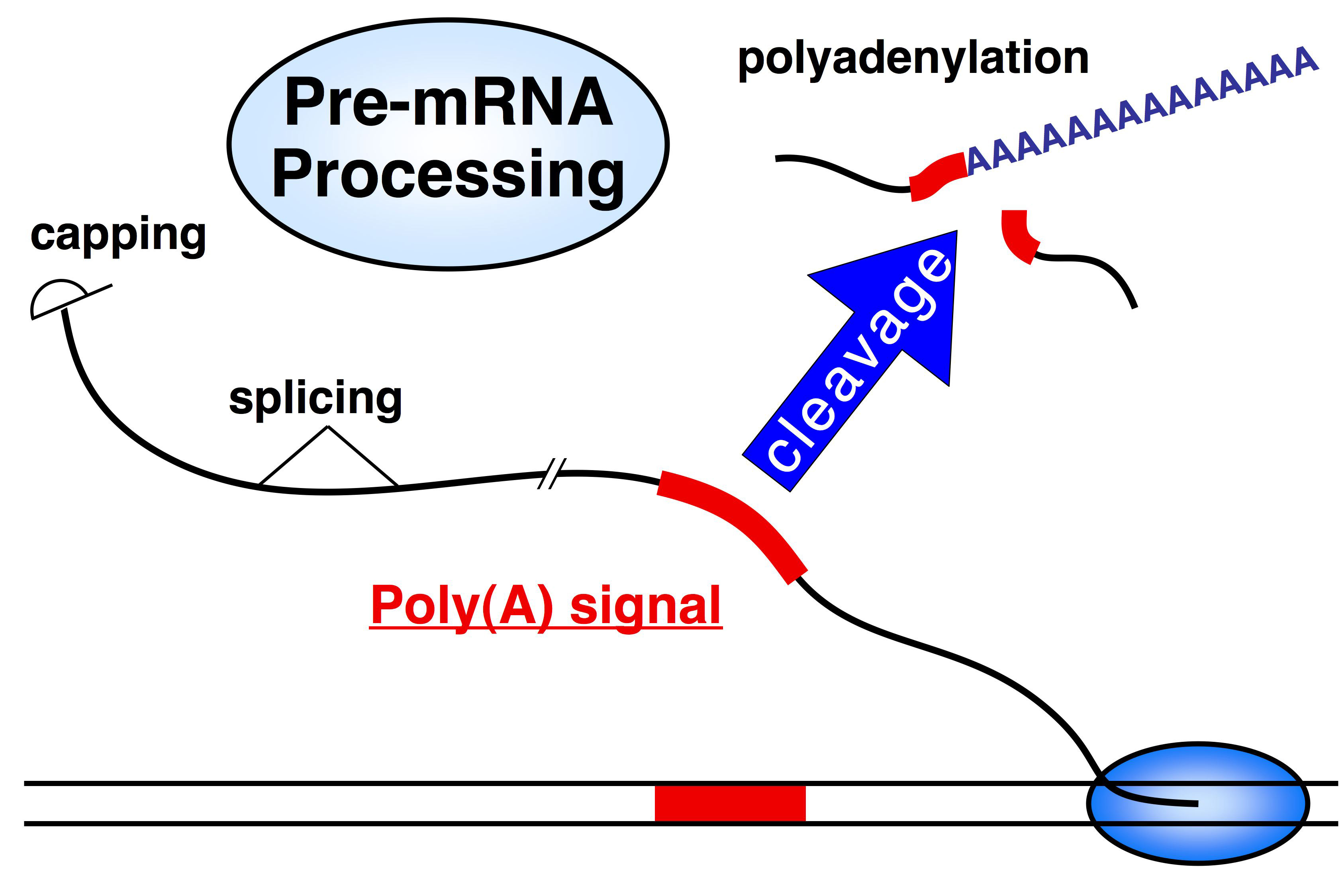
Eukaryotic transcription: termination & polyadenylation
Definition:
This process involves cutting the 3′ end of the new RNA and adding a long string of adenines (A’s) — the poly-A tail.
When It Happens:
Right before transcription ends — after the RNA is made but before RNAP II stops completely.
Key Proteins Involved:
CstF (Cleavage stimulation factor):
Binds to the poly-A signal in the RNA
Cuts the RNA to release the 3′ end
CPSF (Cleavage and polyadenylation specificity factor):
Binds the RNA and recruits PAP
PAP (Poly-A Polymerase):
Adds the poly-A tail (~200 A’s) to the cut 3′ end
Functions of the Poly-A Tail:
Protects the RNA from being degraded
Helps other proteins bind — useful for export and translation
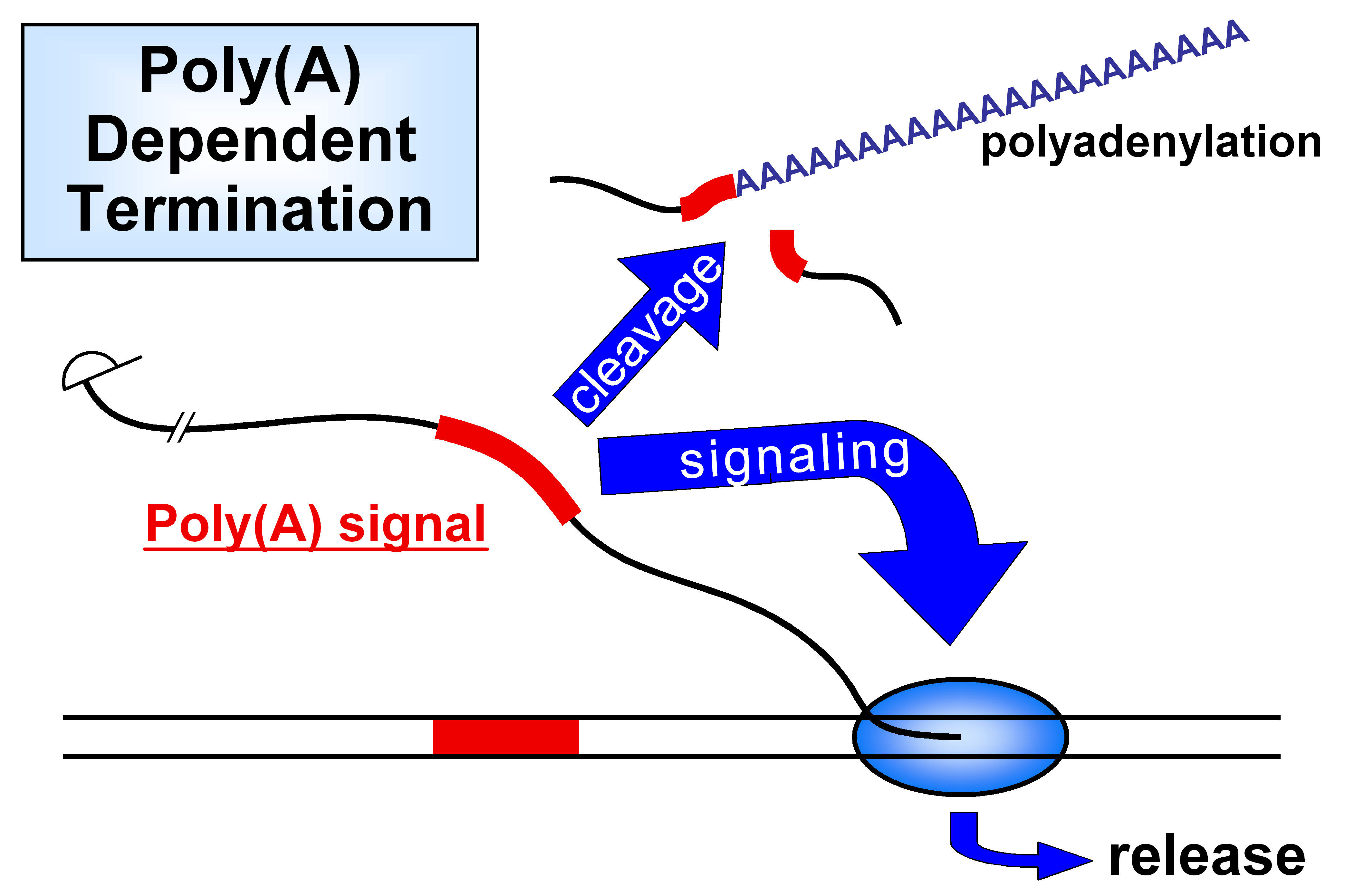
Eukaryotic Transcription: termination
When does termination occur?
After the RNA transcript is released from RNA Polymerase II (RNAP II).
What facilitates termination?
The uncapped 5′ end of the leftover RNA.
This RNA is degraded by RNase, which eventually catches up to RNAP II and knocks it off the DNA.
Torpedo Model:
An exonuclease acts like a "torpedo," degrading RNA from the 5′ end and physically dislodging RNAP II from the DNA template.
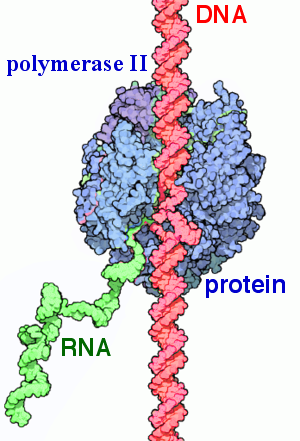
RNAP (RNA Polymerase)
the enzyme that synthesizes RNA from a DNA template during transcription. It binds to DNA at promoter regions and unwinds the helix to catalyze RNA synthesis in the 5’ to 3’ direction.
RNAP Subunits β, β’, α, ω
The core bacterial RNA polymerase consists of five subunits: two α (alpha) subunits for enzyme assembly and DNA interaction; β (beta) and β′ (beta prime) for forming the active site and catalyzing RNA synthesis; and ω (omega), which helps in enzyme assembly and stability.
RNAP σ (sigma) subunit
a detachable component of bacterial RNA polymerase that guides the core enzyme to specific promoter sequences, enabling accurate transcription initiation. Without it, RNAP cannot recognize promoter regions.
σ⁷⁰ (sigma 70) subunit
the primary or “housekeeping” sigma factor in E. coli, responsible for initiating transcription of most essential genes during normal growth conditions.
σᴱ (sigma E) subunit
a stress-response sigma factor activated during envelope stress, such as misfolded proteins in the periplasm. It directs transcription of genes involved in repairing and maintaining the bacterial cell envelope.
σˢ (sigma S) subunit
the stationary phase sigma factor, which becomes active under stress conditions like nutrient starvation or oxidative stress. It promotes transcription of genes that help the cell survive under harsh conditions.
σᴴ (sigma H) subunit
the heat shock sigma factor that directs RNA polymerase to transcribe genes involved in responding to elevated temperatures, particularly those encoding chaperones and proteases that manage protein misfolding.
Rho
a helicase protein involved in Rho-dependent transcription termination in bacteria. It binds to the nascent RNA and travels along it until it catches up to RNA polymerase, where it unwinds the RNA-DNA hybrid, causing transcription to stop.
TRCF (Transcription-Repair Coupling Factor)
a protein that identifies RNA polymerase stalled at DNA lesions and removes it, allowing the nucleotide excision repair (NER) machinery to access and repair the damaged DNA.
RNA Polymerase I (Pol I)
a eukaryotic enzyme that transcribes ribosomal RNA (rRNA) genes, producing a large precursor that is processed into the 18S, 5.8S, and 28S rRNAs (excluding 5S rRNA), all essential components of the ribosome.
RNA Polymerase II (Pol II)
the eukaryotic enzyme responsible for synthesizing messenger RNA (mRNA), small nuclear RNAs (snRNAs), and microRNAs (miRNAs). It plays a central role in gene expression by transcribing protein-coding genes.
RNA Polymerase III (Pol III)
transcribes small structural and functional RNAs in eukaryotes, including transfer RNAs (tRNAs), 5S rRNA, and other small RNAs involved in protein synthesis and regulation.
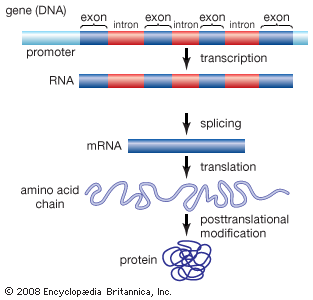
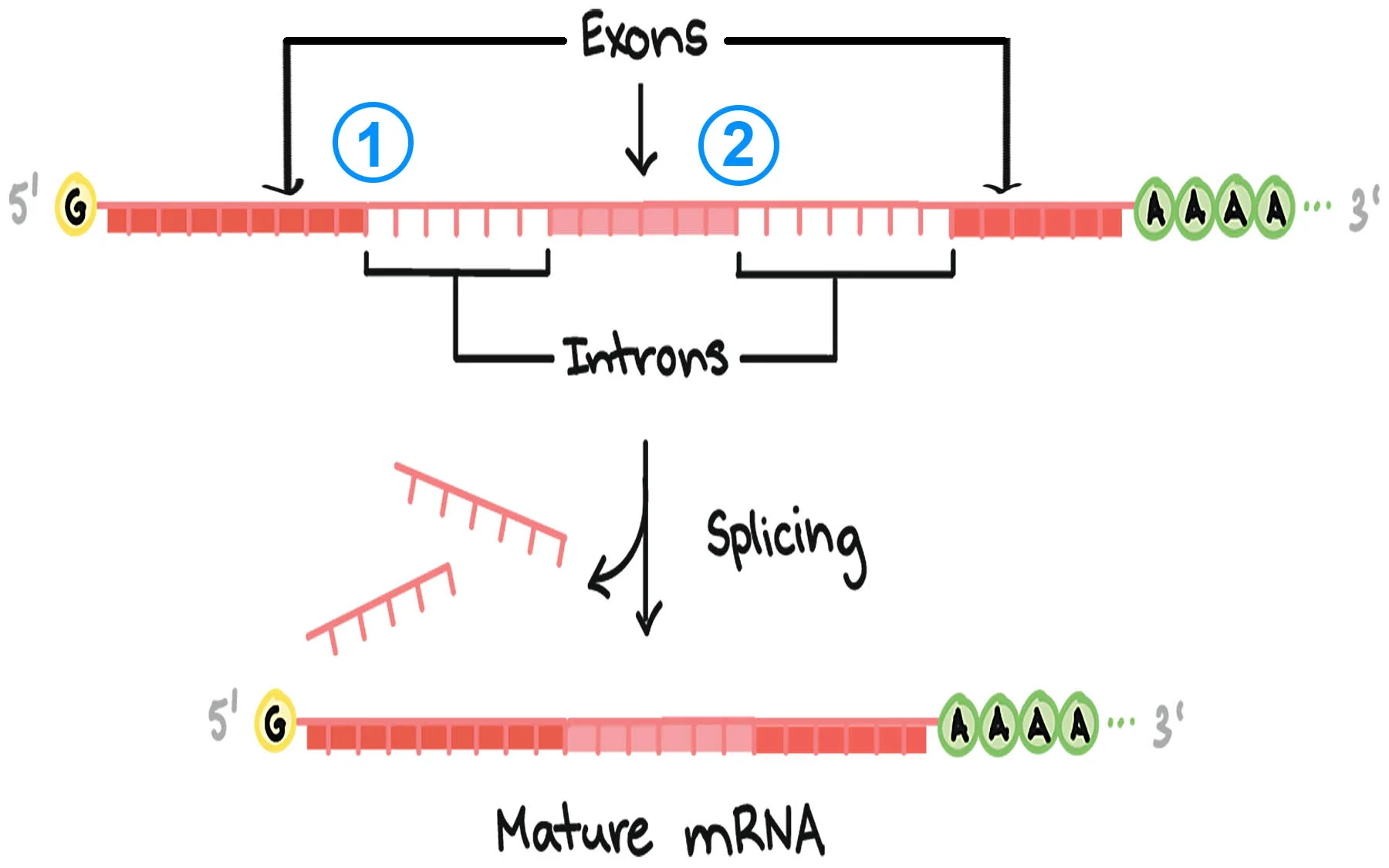
Exon
any RNA sequence, coding or noncoding that is retained in the spliced mRNA “mature mRNA” & thus leaves the nucleus
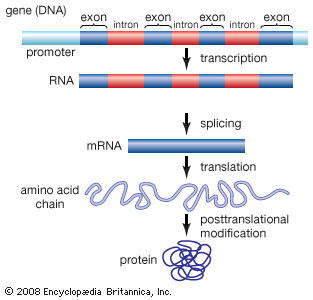
Intron
that is removed before the mRNA leaves the nucleus
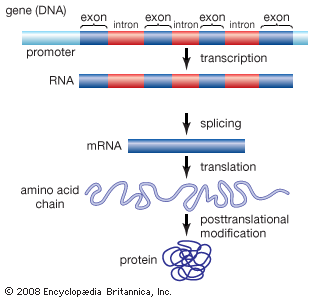
Introns & exons
-eukaryotes, but not every gene & some eukaryotes don’t have any/many
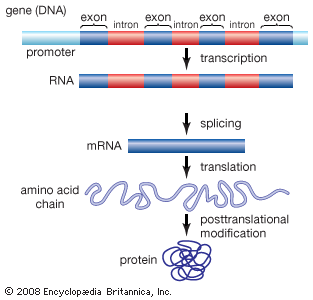
No introns
prokaryotes—>but do have some “self-splicing” introns
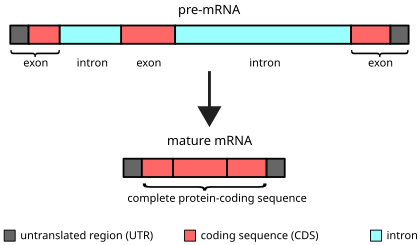
Interesting factors about introns
Definition:
Introns are non-coding sequences found within genes that are removed during RNA processing.
Key Facts:
# of Introns per Gene:
Generally increases with the complexity of the organism.
Size of Introns:
Usually much larger than exons.
Can range from 150 to 800,000 nucleotides (nt) in length.
Introns in Primary RNA (pre-mRNA):
In many genes, most of the RNA is introns, not exons.
Extreme Examples:
Mammalian DHFR gene:
Pre-mRNA = ~31,000 nt
Mature mRNA = ~2,000 nt
Human Dystrophin gene:
Gene size = ~2.4 million nt (2,400 kb)
RNAP makes RNA at ~40 nt/sec → ~17 hours to transcribe this one gene!
Evolutionary Implications of RNA splicing
Definition:
RNA splicing may have evolved to offer protection, adaptability, and increased diversity in gene expression.
Key Theories:
Transposon Defense Theory:
Introns may have come from transposons (jumping genes).
Early transposons harmed cells, so cells evolved splicing to remove them from RNA before they caused damage.
Mutation Buffer Theory:
Introns may help protect genes by absorbing mutations, making harmful changes less likely to affect essential coding regions.
Exon Shuffling Theory:
Introns allow for recombination between exons, helping create new proteins with mixed functional domains — increasing genetic diversity.
Evolutionary Advantage:
Alternative Splicing:
A single gene can produce multiple proteins by splicing RNA in different ways.
This increases protein variety without needing more genes — a big evolutionary win!
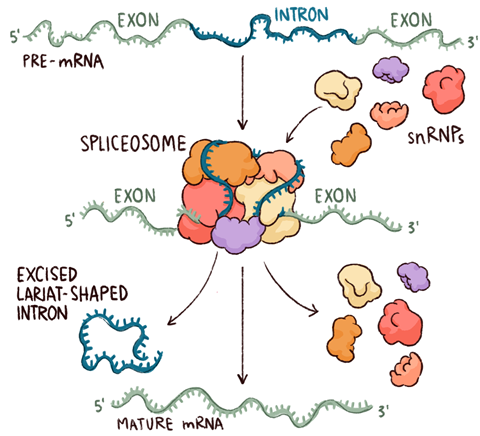
Intron splicing classes
Introns (non-coding RNA sequences) are removed from pre-mRNA using three different splicing mechanisms, or classes.
1. Spliceosome-Mediated Splicing:
Used by the majority of eukaryotic genes
Involves a large complex called the spliceosome (made of proteins + snRNAs)
Most common splicing mechanism
2. Group II Intron Self-Splicing:
Introns fold into a specific shape that allows them to splice themselves out
Found in some eukaryotic organelle genes (like mitochondria/chloroplasts) and some prokaryotes
Rare
3. Group I Intron Self-Splicing:
Also self-splicing, but uses a free guanine as a helper (a "tool")
Found in nuclear rRNA genes and some prokaryotic genes
Also rare
Intron splicing via spliceosome
RNA sequence mediated:
-splicing is mediated by RNA sequences in the intron & exon
5’ splice site:
-at the 5’ end —> need a GU
3’ splice site:
-at the 3’ end: need an AG
Branch site:
-conserved adenine
-very few sequence requirements
1) spliceosome helps control the process —> splicing at correct sites
2) often get mis-spliced
TBP (TATA-binding protein)
A component of the transcription factor TFIID that binds to the TATA box in eukaryotic promoters, helping to position RNA polymerase II for transcription initiation.
Mediator complex
A large multi-protein complex that acts as a bridge between transcription factors and RNA polymerase II, facilitating transcription initiation and regulation.
Elongation factors
Proteins that assist RNA polymerase or ribosomes during the elongation phase of transcription or translation by enhancing processivity and accuracy.
CstF (Cleavage stimulation factor)
A protein complex involved in 3' end processing of pre-mRNA; it recognizes and binds downstream of the polyadenylation signal to promote mRNA cleavage.
CPSF (Cleavage and polyadenylation specificity factor)
A multi-subunit protein complex that recognizes the AAUAAA signal in pre-mRNA and works with CstF to cleave the transcript and initiate polyadenylation.
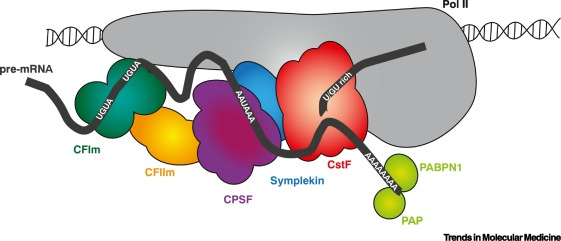
PolyA Polymerase (PAP)
An enzyme that adds a polyadenine (poly-A) tail to the 3' end of a newly cleaved pre-mRNA, important for transcript stability and export from the nucleus.
Spliceosome complex
A dynamic RNA-protein complex that removes introns from pre-mRNA and ligates exons together during RNA splicing.
Aminoacyl-tRNA synthetase
An enzyme that attaches the appropriate amino acid to its corresponding tRNA molecule, a critical step for accurate translation.
Ribosomal large subunit
The larger part of the ribosome (60S in eukaryotes, 50S in prokaryotes) that catalyzes peptide bond formation during translation.
Ribosomal small subunit
The smaller part of the ribosome (40S in eukaryotes, 30S in prokaryotes) that binds to mRNA and is responsible for decoding the genetic message.
Initiation Factor 1 (IF1)
A bacterial translation initiation factor that blocks the A site on the small ribosomal subunit to prevent premature tRNA binding.
Initiation Factor 2 (IF2)
A bacterial GTPase that facilitates the binding of the initiator tRNA (fMet-tRNA) to the start codon on the small ribosomal subunit.
A bacterial GTPase is a type of enzyme found in bacteria that binds and hydrolyzes GTP. GTPases act like molecular switches, turning cellular processes on or off depending on whether they’re bound to GTP (active) or GDP (inactive).
Initiation Factor 3 (IF3)
A bacterial initiation factor that prevents premature joining of the large and small ribosomal subunits and helps position mRNA on the small subunit.
Class I Release Factors
Proteins (e.g., RF1 and RF2 in bacteria) that recognize stop codons in the A site of the ribosome and promote the release of the newly synthesized polypeptide.
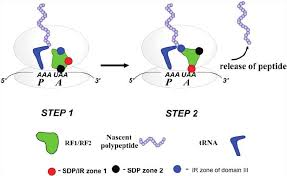
Class II Release Factors
GTP-binding proteins (e.g., RF3 in bacteria) that assist Class I release factors in terminating translation by accelerating their release from the ribosome.
Chemistry of splicing
Spliceosome mediated splicing
1) 2’ OH of adenosine in branch site:
-attacks the phosphodiester bond between the upstream exon & the G at the 5’ end of the intron
2) 3’ OH of 1st exon:
-attacks phosphodiester bond linking the intron to the downstream exon
-no net creation or destruction of bonds but uses a lot of ATP
this is used to “control the rxn”
Spliceosomes
A huge molecular machine that removes introns from pre-mRNA in eukaryotic cells — often described as looking like a "boxing glove" or "oven mitt."
General Structure:
Made of >150 proteins
Contains 5 small nuclear RNAs (snRNAs)
Together, these form snRNPs (pronounced “snurps”):
Small Nuclear RiboNuclear Proteins
snRNAs:
About 100–300 nucleotides long
Form complexes with proteins to help build the spliceosome
Roles of snRNPs ("snurps"):
Recognize the 5′ splice site and the branch point A
Bring the splice sites together to set up the reaction
Catalyze (or help catalyze) the two cut-and-paste splicing reactions
Self-splicing RNAs (are they ribozymes?)
Yes, meaning RNA molecules that act like enzymes to catalyze their own splicing (no proteins needed).
Key Concept – Self-Splicing:
Some introns can fold into a shape that allows them to remove themselves from RNA.
Group I Introns:
Use a free guanine (G) to start splicing.
3′ OH of guanine attacks the 5′ splice site.
Chemistry is similar to the spliceosome, just done without protein help.
Group II Introns:
More complex but similar to spliceosome action.
Steps:
Guanine-binding pocket holds a free G.
3′ OH of G attacks the 3′ end of upstream exon (which is on the 5′ end of the intron).
The 3′ OH of the upstream exon then attacks the 5′ end of the downstream exon, joining the exons together.
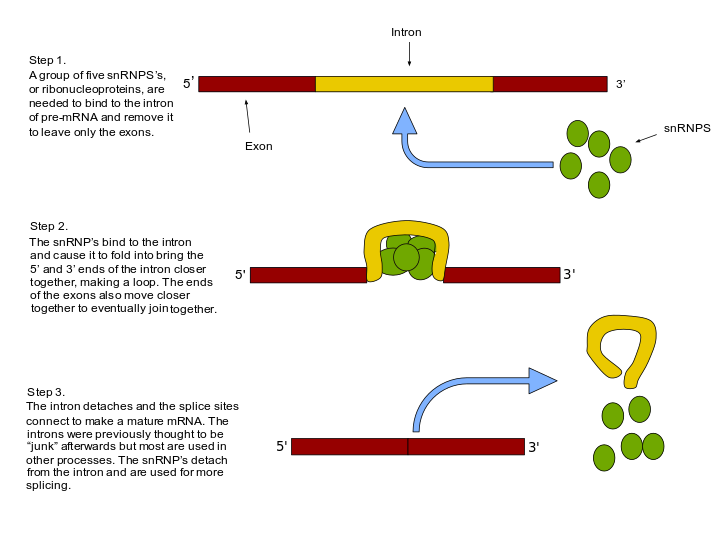
Splicing errors (& how they are avoided)
can lead to incorrect mRNA and nonfunctional proteins. Cells have mechanisms to ensure accurate splicing.
Common Splicing Errors:
Exon Skipping:
A mistake where the spliceosome accidentally skips over an exon and joins non-adjacent exons.
Pseudo Splice-Site Selection:
The spliceosome chooses a wrong (fake) splice site, leading to faulty splicing.
Mechanisms to Ensure Splicing Accuracy (Splicing Fidelity):
Co-transcriptional Loading:
Splicing proteins (like snRNPs) are recruited during transcription, helping splice the RNA as it’s being made — reduces chances for mistakes.
Splicing Guides:
Special polar and basic proteins bind to exon regions and help guide correct splicing.
Exonic Splicing Enhancers (ESEs):
Short sequences within exons that attract splicing factors to ensure proper exon recognition and inclusion.
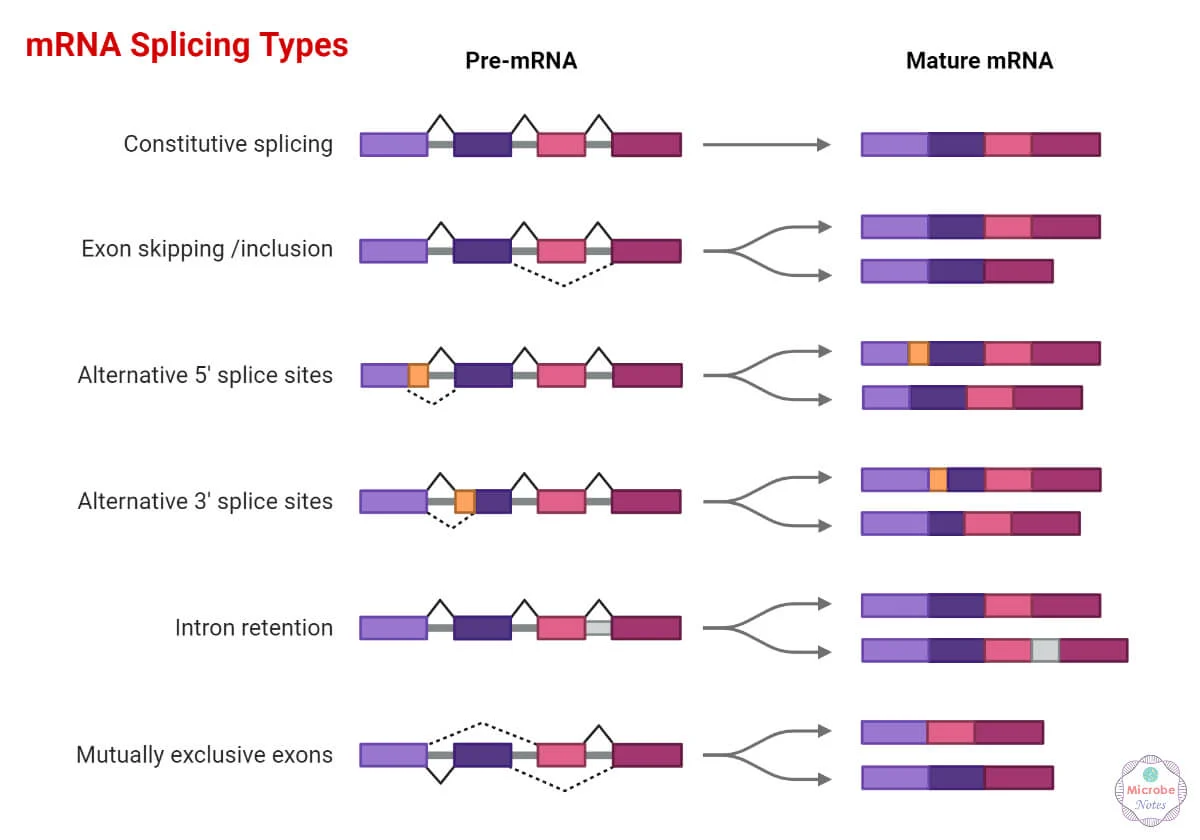
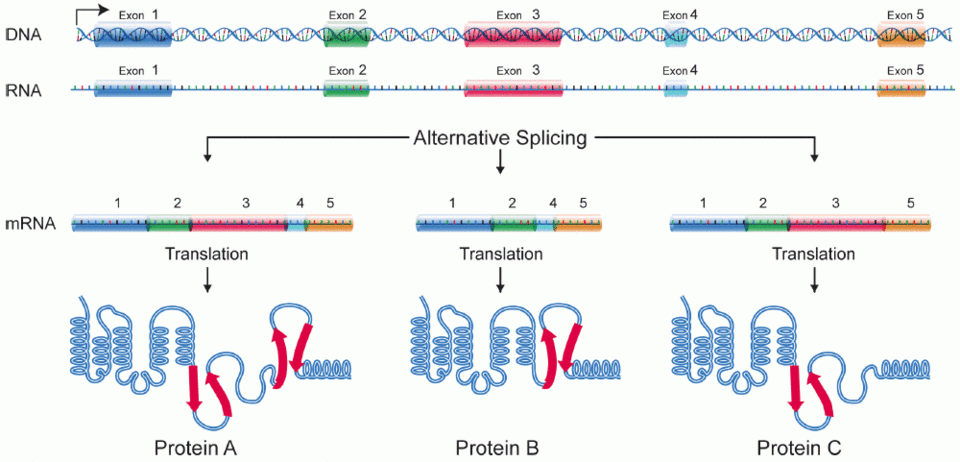
Alternative splicing
A process where one gene can produce multiple mature mRNAs, each coding for different proteins (called RNA isoforms).
Key Points:
It’s a regulated process, not a mistake.
Controlled by splicing enhancers (promote splicing) and silencers (block splicing).
Allows cells to make many proteins from a single gene — increases diversity.
Examples:
Human S10 gene → over 100 isoforms
Drosophila dscam gene → over 1,000 isoforms
Fun Fact:
Alternative splicing occurs in about 70% of human genes!
Interesting splicing phenomena
Trans-splicing:
-when two exons from different pre-mRNA are spliced together
More interesting RNA modifications
A3G (APOBEC3G) is a special enzyme (a cytidine deaminase) that edits viral RNA, especially HIV RNA.
How It Works:
A3G changes cytosine (C) to uracil (U) in HIV RNA.
This causes mutations in the viral genome, often creating premature stop codons → makes the virus nonfunctional.
HIV’s Defense:
HIV produces a protein called Vif (Virion infectivity factor).
Vif inactivates A3G, allowing HIV to escape this antiviral editing and continue replicating.
Why It Matters:
A3G is part of the body's innate immune defense against viruses, especially retroviruses like HIV.
Translation
the process of building proteins using the instructions in mRNA.
Key Points:
High energy cost – especially in fast-growing cells like E. coli, which uses up to 80% of its energy on translation.
Main components involved:
mRNA – the instructions (messenger RNA)
tRNA – brings amino acids to the ribosome
Ribosome – the site where proteins are made
Amino acids – the building blocks of proteins
Bonus term:
Aminoacyl-tRNA synthetase – enzyme that attaches the correct amino acid to its matching tRNA.
Note:
Water (H₂O) is released when amino acids are joined together to form peptide bonds during translation (a dehydration reaction).
The messenger RNA
mRNA carries the genetic instructions from DNA to the ribosome, where it’s translated into protein.
Key Terms:
ORF (Open Reading Frame):
A continuous stretch of codons that could be translated into a protein. If it actually makes a protein, it’s considered a gene.Monocistronic mRNA:
Found in eukaryotes
mRNA that codes for one protein
Polycistronic mRNA:
Found in prokaryotes
mRNA that codes for multiple proteins, usually related in function (like part of an operon)
Sites of translation initiation
In prokaryotes:
-need ribosome binding site (RBS)
In eukaryotes:
-ribosome binds the 5’ CAP & scans downstream for a start codon
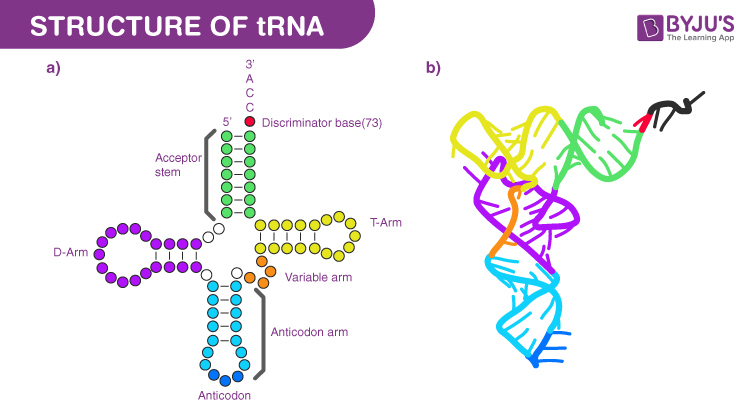
transfer RNAs (tRNAs)
Definition:
tRNAs are small RNA molecules that deliver the correct amino acid to the ribosome during translation.
Main Role:
Each tRNA matches a codon in the mRNA with its specific amino acid, bringing it to the ribosome's active site for protein building.
Common Structural Features:
Rigid shape – helps maintain proper alignment in the ribosome
Cloverleaf structure – 2D shape formed by internal base pairing
Acceptor arm – found at the 3′ end, where the amino acid is attached
Unusual (Modified) Nucleotides:
tRNAs often contain chemically modified bases, which help with folding, stability, and function.
Examples:
Pseudouridine (Ψ)
Dihydrouridine (D)
Hypoxanthine (found in inosine)
Thymine (even though it's usually only in DNA!)
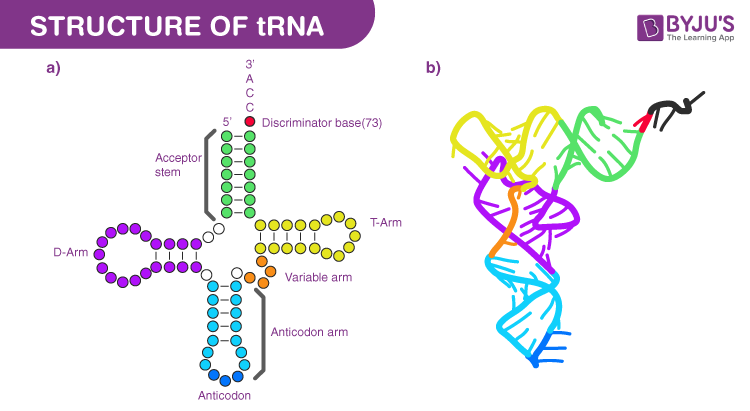
tRNA structure
tRNA (transfer RNA) acts as a bridge between mRNA codons and amino acids, linking nucleic acid information to protein building.
Key Structural Features:
Anticodon Loop:
Contains a three-nucleotide sequence (anticodon) that pairs with the codon on mRNA.
Acceptor Arm:
Located at the 3′ end of the tRNA
Holds the amino acid that will be added to the growing protein chain.
Main Function:
tRNA connects the genetic code (RNA) to the correct amino acid, making it essential for accurate translation.
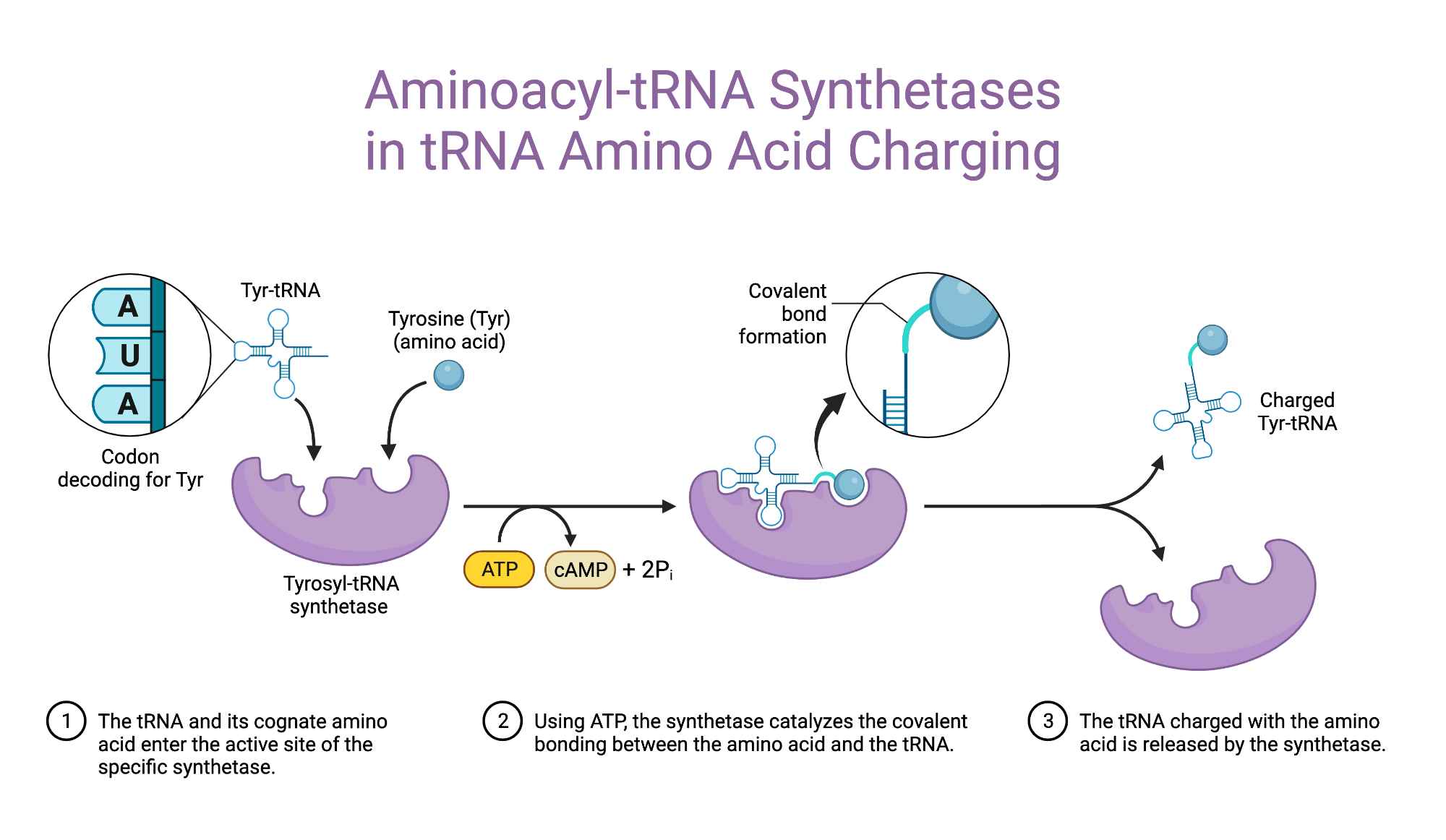
Aminoacyl-tRNA synthetase
An enzyme that "charges" tRNA by attaching the correct amino acid to the acceptor arm of a tRNA molecule using a covalent bond.
Key Point:
Charged tRNA: Has an amino acid attached → ready for translation.
Uncharged tRNA: No amino acid attached → not yet usable by the ribosome.
Why it matters:
This enzyme ensures that the correct amino acid is matched with the correct tRNA — essential for accurate protein synthesis.
Steps in charging of tRNA
Charging a tRNA means attaching the correct amino acid to it — a crucial step for accurate translation.
Step 1: Adenylation of the Amino Acid
The enzyme uses ATP to activate the amino acid:
Amino Acid + ATP → aminoacyl-AMP + pyrophosphate (PPi)
Step 2: Transfer to tRNA
The activated amino acid is then transferred to the tRNA:
Aminoacyl-AMP + tRNA → aminoacyl-tRNA + AMPNow the tRNA is “charged” and ready for the ribosome.
Accuracy:
Very high – only 1 error in every 10,000 (10⁴) reactions
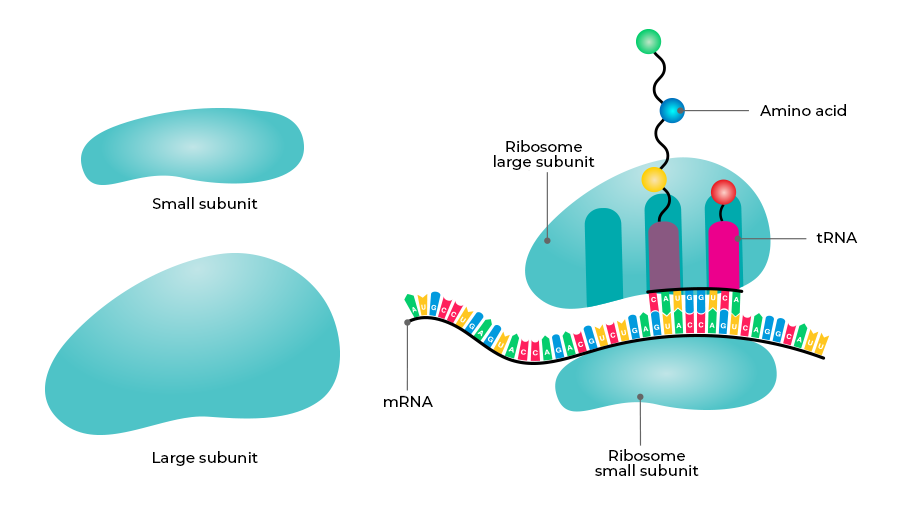
Ribosome
Definition:
a complex made of ribosomal RNA (rRNA) and proteins that builds proteins from mRNA.
Key Points:
Structure:
Made of rRNA (does most of the work) and proteins (support roles) — similar to the spliceosome.
Subunits:
Small subunit: Decodes the mRNA by matching codons to tRNA anticodons.
Large subunit: Forms peptide bonds between amino acids (called the peptidyl transfer center).
Terminology:
Named by how they sediment (settle) during centrifugation — measured in Svedberg (S) units, not additive.
Prokaryotic Ribosome:
Small: 30S
Large: 50S
Total: 70S
Eukaryotic Ribosome:
Small: 40S
Large: 60S
Total: 80S
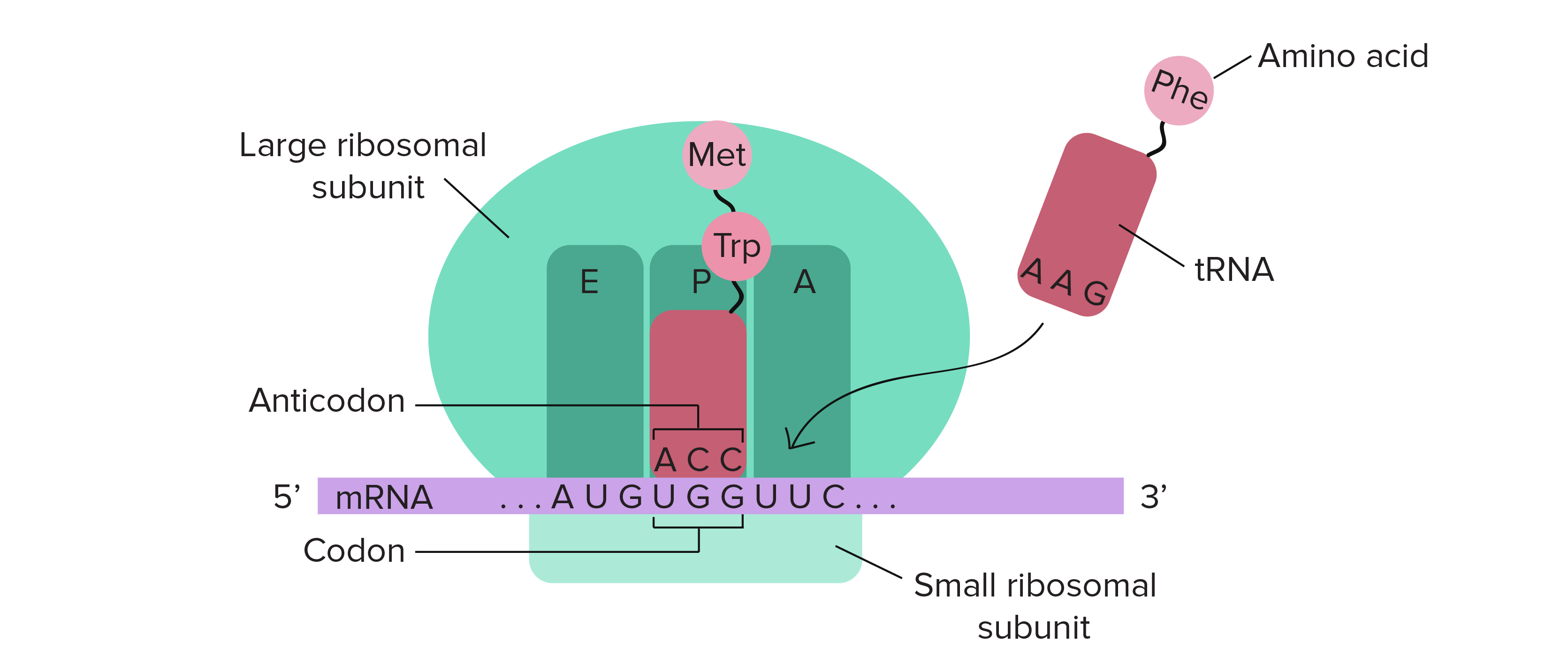
Ribosome: sites
3 sites to know
A site: where the charged aminoacyl tRNA binds
P site: where the peptidyl-tRNA binds
E site: where the uncharged-tRNA exits
Stages of translation
Initiation: translation stars, highly regulated
Elongation: translation continues, adding one AA to polypeptide as the aminoacyl-tRNA moves to the P site
Termination: translation ends
-ribosome releases the mRNA & polypeptide
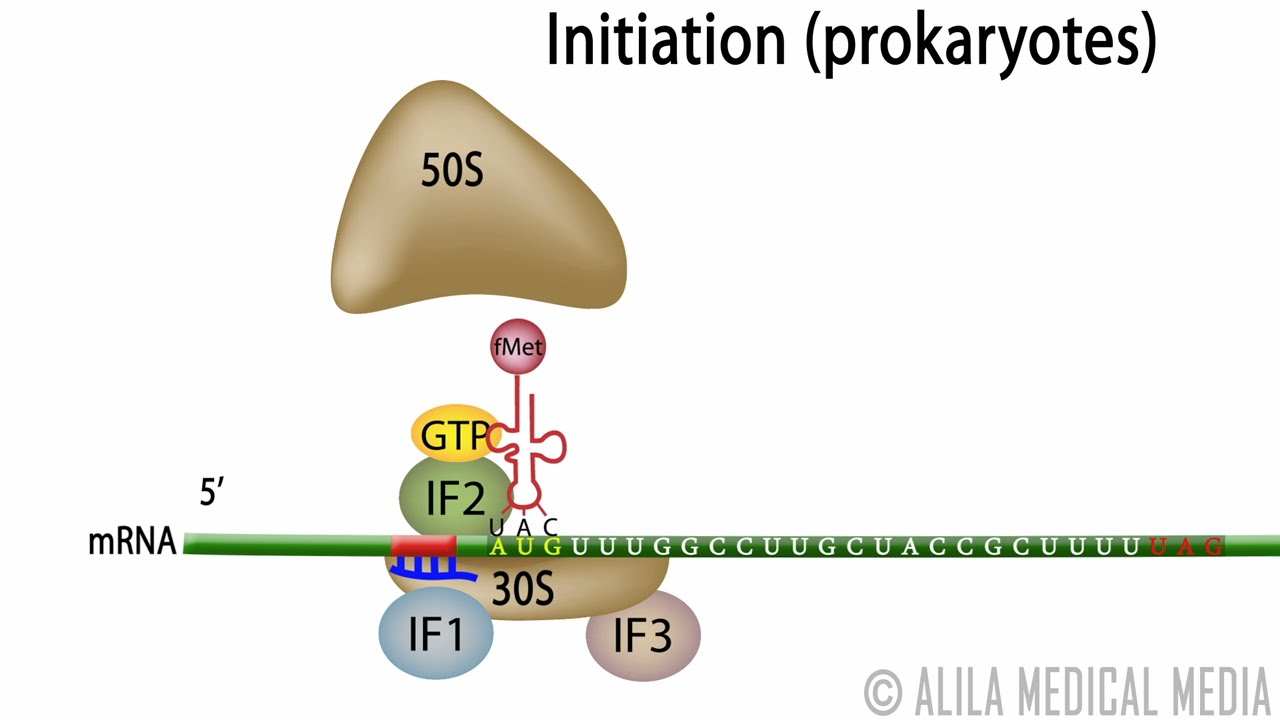
Translation initiation: prokaryotes
Definition:
The process that sets up the ribosome to begin translating an mRNA into protein in bacteria like E. coli.
Initial State:
The large (50S) and small (30S) ribosomal subunits are separate.
Step-by-Step Process:
IF3 binds to the E site of the small subunit
Prevents large and small subunits from joining too early.
IF1 binds the A site
Blocks tRNA from entering too soon.
IF2 binds GTP and positions near the P site to help bring in the starting tRNA.
The 30S subunit + IFs bind:
the mRNA
and the initiator tRNA (fMet-tRNA)
IF3 leaves, allowing the 50S large subunit to join.
IF2 hydrolyzes GTP → GDP, triggering the release of IF2 and IF1.
End Result:
A complete 70S initiation complex is formed.
The P site holds the first tRNA, and the A site is ready for the next tRNA to enter.
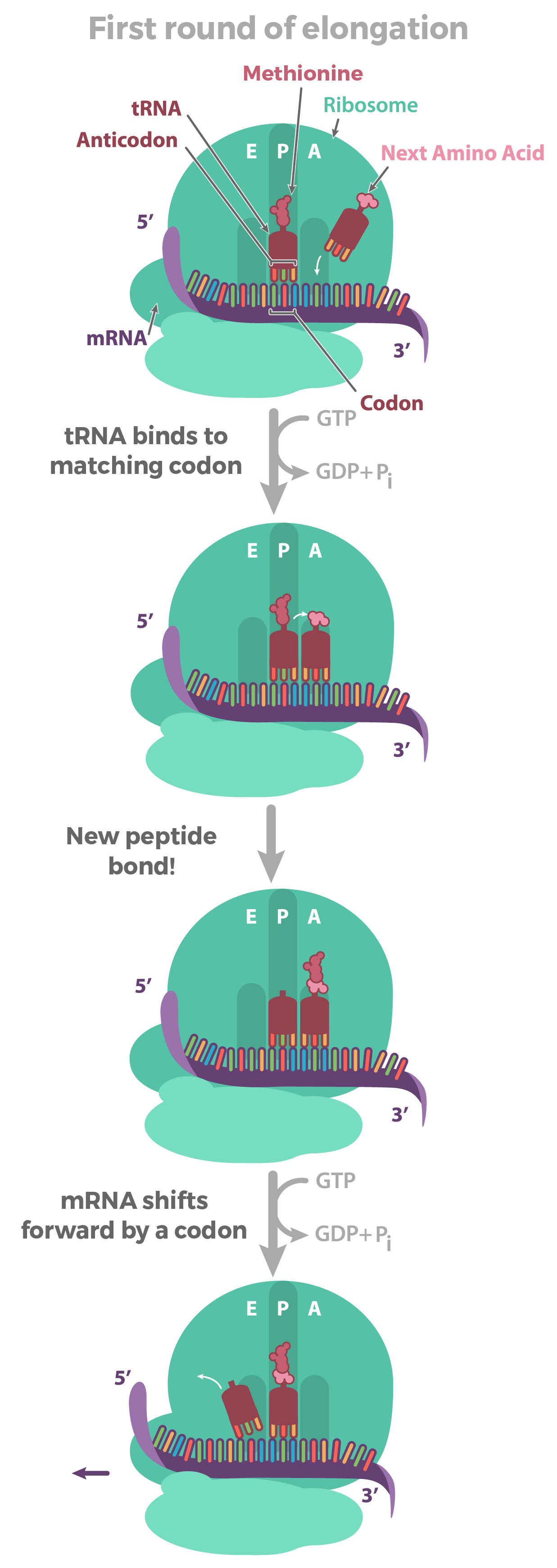
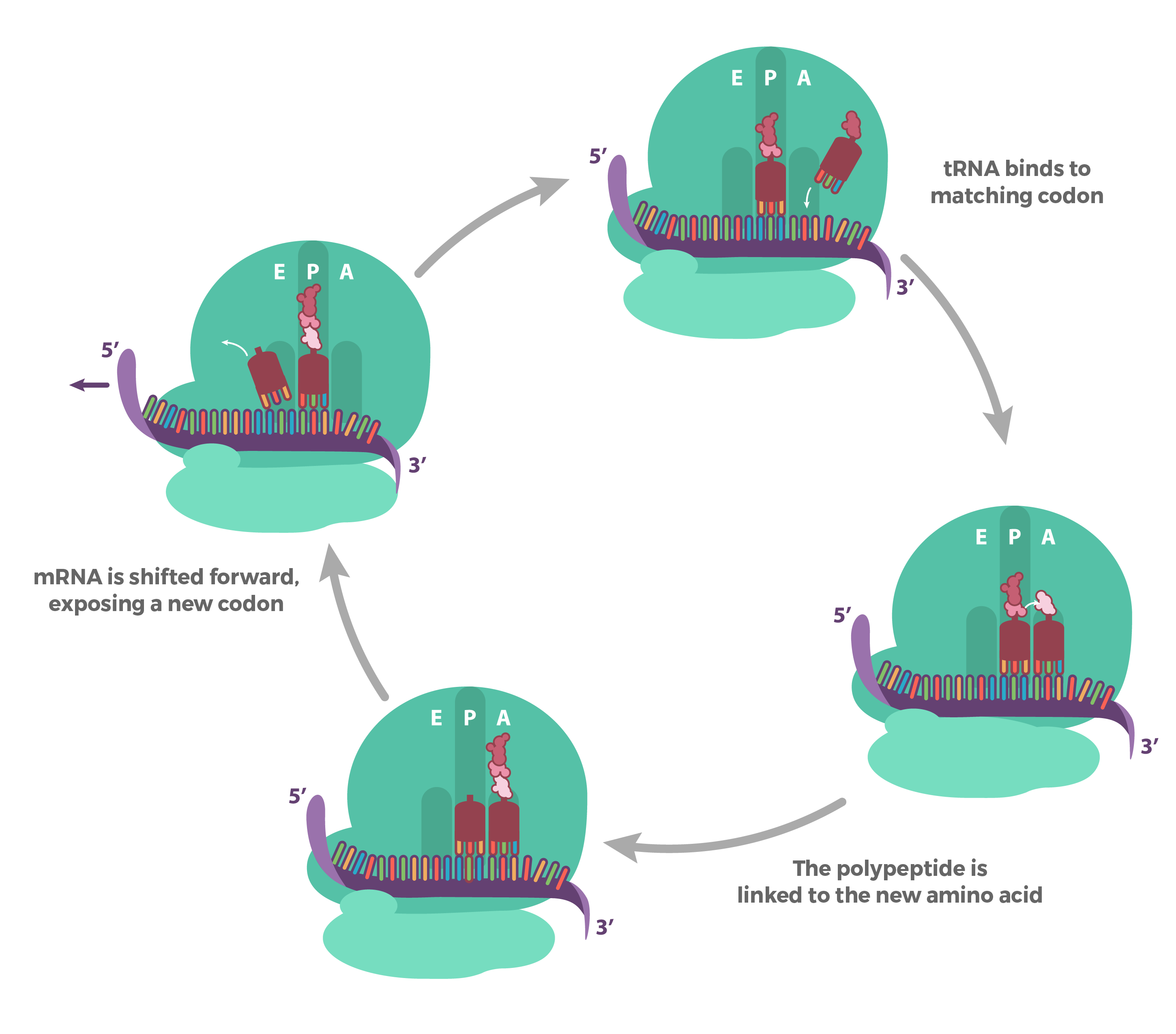
Translation elongation
Prokaryotes vs eukaryotes: bascially the same!
The process:
1) tRNA (charged) binds the codon in the A site
2) large subunit catalyzes peptide bond formation of nascent peptide on peptidyl-tRNA (in P site) to AA on aminoacyl-tRNA in A site As aminoacyl moves from A site to P site
3) Uncharged tRNA leaves E site
Translation termination
Translation ends when the ribosome reaches a stop codon on the mRNA.
Key Concepts:
Stop Codon Recognition:
There are no tRNAs that match stop codons (UAA, UAG, UGA).
Instead, release factors handle termination.
Release Factors:
Class I Release Factors (e.g., RF1, RF2):
Bind directly to the stop codon in the A site.
Help release the newly made protein from the P site.
Class II Release Factors (e.g., RF3):
Help remove Class I release factors from the ribosome using GTP hydrolysis.
After Termination:
The ribosome disassembles:
The large and small subunits separate.
The mRNA is released.
Special Case – In Some Bacteria (like E. coli):
Under certain conditions, ribosomes may bypass the stop codon and resume translation at the next AUG — called translational recoding or reinitiation.
RNA polymerase structure
a multi-subunit enzyme with a core made of α, β, β′, and ω subunits in bacteria, and additional subunits in eukaryotes. It forms a channel for DNA entry, RNA exit, and nucleotide addition.
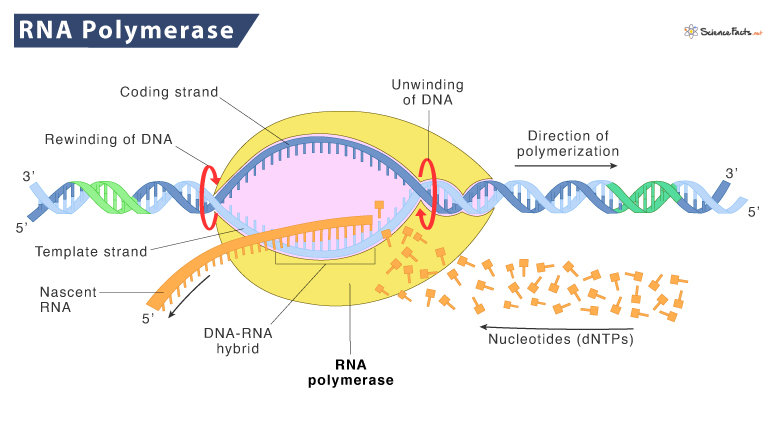
RNA polymerase catalytic mechanism
RNA polymerase adds ribonucleotides to the 3′ end of a growing RNA strand using a DNA template; this occurs through a two-metal ion mechanism that facilitates phosphodiester bond formation.
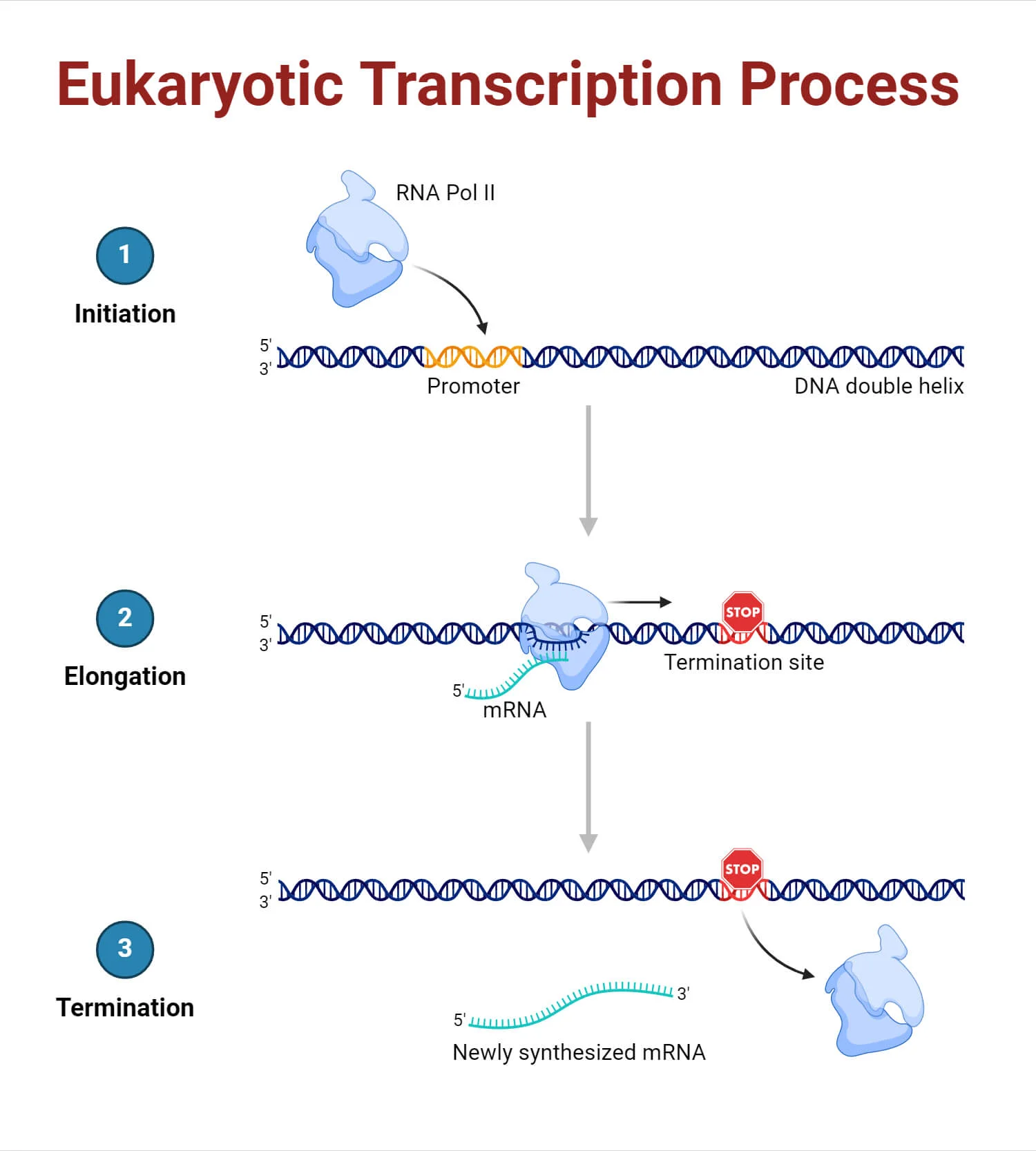
3 main steps of transcription
1) Initiation – RNA polymerase binds to the promoter; 2) Elongation – RNA strand is synthesized; 3) Termination – RNA synthesis stops and the RNA is released.
Bacterial promoters
DNA sequences upstream of a gene where RNA polymerase binds; key elements include the −10 (Pribnow box) and −35 regions recognized by the σ factor.
Sigma (σ) factors
Specialized bacterial protein subunits that guide RNA polymerase to specific promoter sequences and help initiate transcription.
Bacterial transcription: initiation
Involves RNA polymerase holoenzyme (core + σ factor) recognizing the promoter, unwinding DNA, and beginning RNA synthesis.
Abortive initiation
A phase in transcription initiation where RNA polymerase repeatedly synthesizes and releases short RNA transcripts (2–9 nucleotides) before transitioning to elongation.
“Escaping the promoter”
The process by which RNA polymerase clears the promoter after abortive initiation and transitions into elongation by breaking interactions with the σ factor.
DNA scrunching mechanism
A model for transcription initiation in which RNA polymerase pulls downstream DNA into itself while the enzyme remains stationary, generating stress that helps escape the promoter.
3 mechanisms of bacterial transcription: termination
1) Rho-independent (intrinsic) – relies on a GC-rich hairpin followed by a U-rich region; 2) Rho-dependent – requires the Rho protein; 3) Hybrid mechanisms – involve aspects of both.
3 RNAPs in eukaryotes
1) RNAP I – transcribes rRNA (except 5S); 2) RNAP II – transcribes mRNA and some snRNA; 3) RNAP III – transcribes tRNA, 5S rRNA, and other small RNAs.
RNAP II structure
A 12-subunit complex responsible for transcribing protein-coding genes; it features a mobile clamp and a C-terminal domain (CTD) of repeats crucial for regulation.
Class II promoters
Promoters transcribed by RNAP II; contain a core promoter (e.g., TATA box) and regulatory elements for transcription factor binding.
Eukaryotic pre-initiation complex and promoter escape
A complex of RNAP II and general transcription factors (TFIID, TFIIH, etc.) that assembles at the promoter and facilitates transcription initiation and escape into elongation.
Eukaryotic transcription: elongation
After promoter escape, RNAP II synthesizes RNA as elongation factors assist with processivity and suppress pausing/backtracking.
RNAP II carboxy-terminal tail (CTD)
A repetitive tail on the largest RNAP II subunit (YSPTSPS repeats) that is phosphorylated at specific residues to regulate transcription and RNA processing.
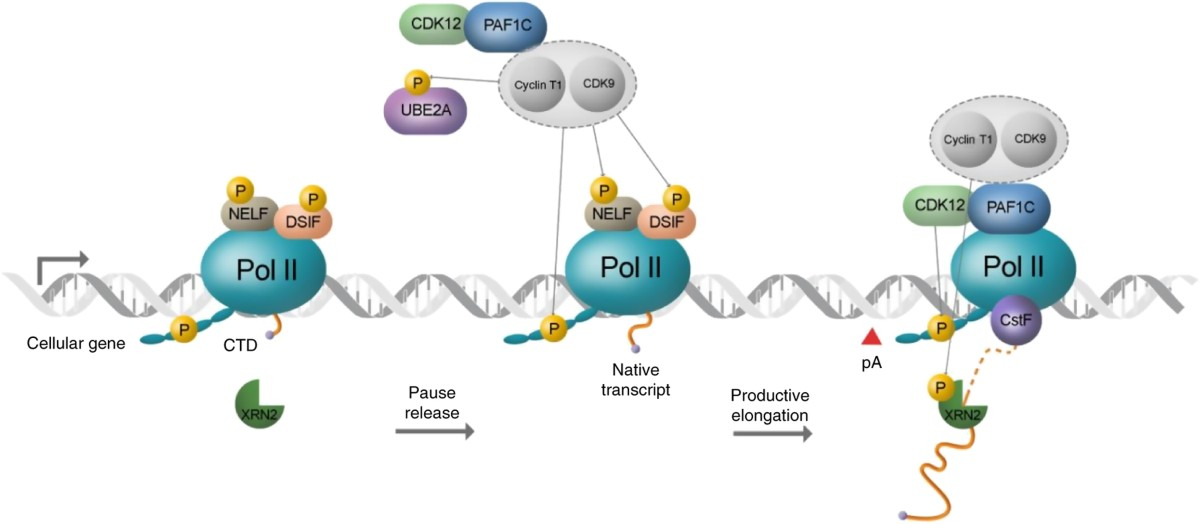
Role of serine phosphorylation of CTD in regulating stages of transcription
Ser5 phosphorylation promotes promoter clearance and capping enzyme recruitment; Ser2 phosphorylation enhances elongation and 3′ end processing.
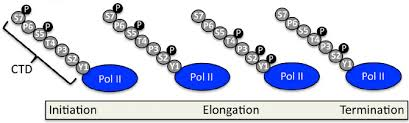
CTD Phosphorylation for Transcription Initiation
At the start of transcription, the CTD (carboxy-terminal domain) of RNAP II is mostly unphosphorylated, allowing the pre-initiation complex to form.
CTD Phosphorylation Right After Initiation
Once transcription begins, Ser5 on the CTD is phosphorylated. This signals promoter escape and helps recruit the capping enzyme.
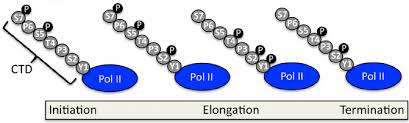
CTD Phosphorylation during elongation
During elongation, Ser2 gets phosphorylated, which helps recruit proteins for splicing and 3′ end processing (like polyadenylation).
RNA Capping – What, How & Why
What: A 7-methylguanosine cap is added to the 5′ end of mRNA.
How: Happens soon after transcription starts (with Ser5 phosphorylation).
Function: Protects mRNA, helps it exit the nucleus, and aids in ribosome binding for translation.
Polyadenylation – What, How & Why
Definition:
What: A poly-A tail (lots of A’s) is added to the 3′ end of mRNA.
How: Cleavage of the RNA occurs first, then poly-A polymerase adds ~200 A’s.
Function: Increases mRNA stability, helps with export and translation.
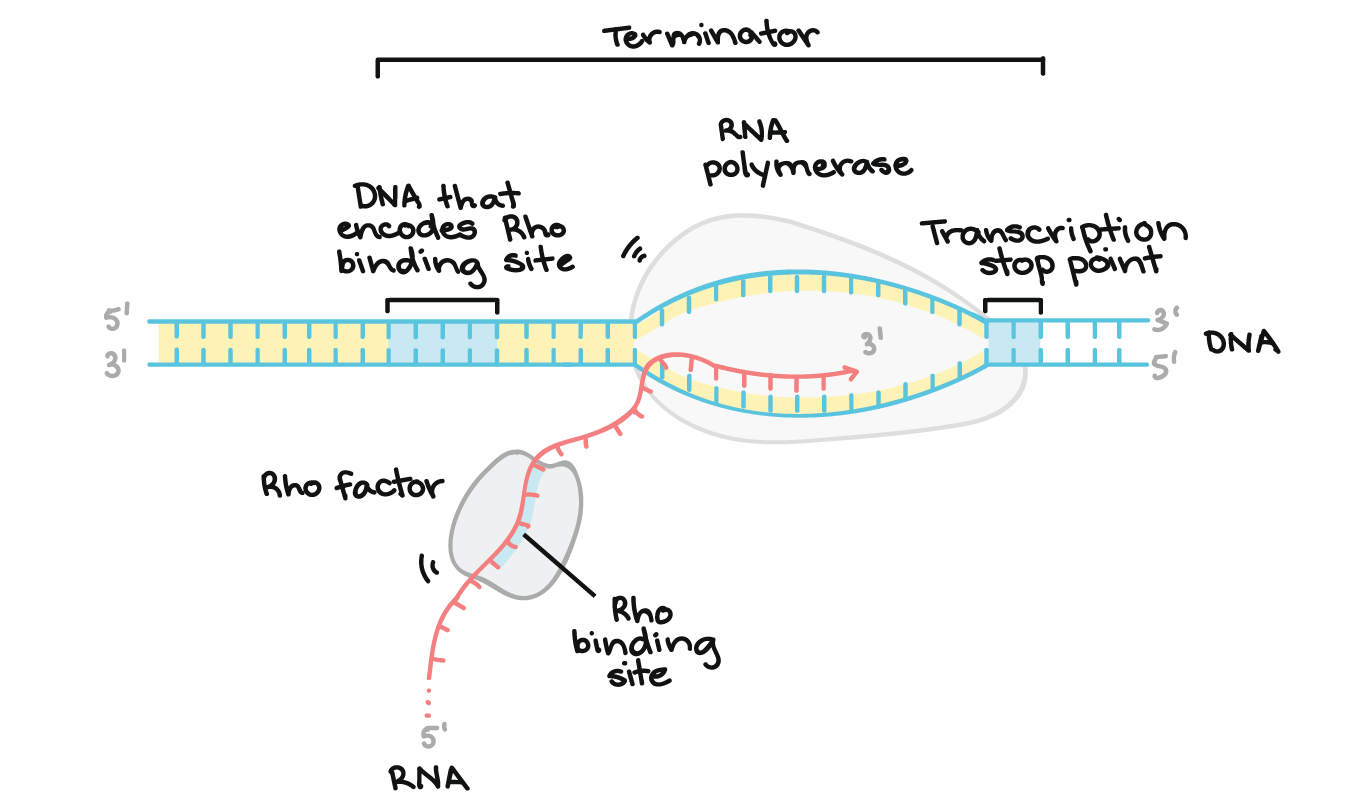
Eukaryotic Transcription Termination
RNA is cleaved near the poly-A site, then RNAP II keeps transcribing. A "torpedo" enzyme (like Xrn2) degrades the leftover RNA, causing the polymerase to fall off.
2 Theories of RNA Splicing Origin
Definition:
Transposon Theory: Introns came from transposons; splicing evolved to remove them.
Protective Theory: Introns evolved to buffer against mutations by keeping important coding areas safe.
Evolutionary Advantage(s) of Splicing
Allows alternative splicing (1 gene → many proteins)
Exon shuffling can create new proteins
Helps with gene regulation and diversity
3 Classes of Splicing Mechanisms
Spliceosome-mediated (most common in eukaryotes)
Group I intron self-splicing
Group II intron self-splicing
Spliceosome-Mediated Splicing
Important Sequences: 5′ splice site (GU), branch point (A), 3′ splice site (AG)
Chemistry: 2 transesterification reactions
Process: snRNPs recognize sites, cut intron, and join exons together
Possible Splicing Errors
Exon Skipping: Wrong exons joined
Pseudo Splice Site Use: Incorrect site is used
→ Can cause nonfunctional or harmful proteins
2 Mechanisms to Promote Accurate Splicing
Co-Transcriptional Splicing: Splicing happens while RNA is being made
Splicing Guides & Enhancers: Proteins and sequences help the spliceosome choose the correct sites
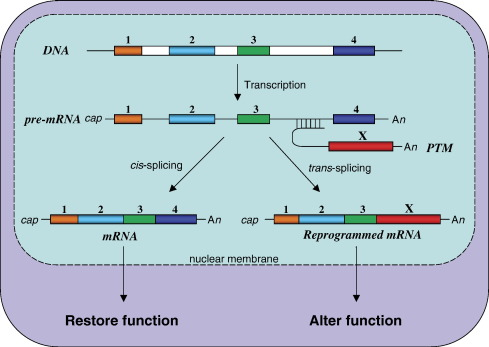
Trans-Splicing
A rare process where exons from two different RNA molecules are joined together. Seen in some protists and worms.
Alternative Splicing
One gene can produce multiple mRNA versions by changing how exons are joined. This creates protein diversity from a single gene.