OCR (A) Chemistry GCSE
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
45 Terms
What are the three states of matter?
Solid
Liquid
Gas
Describe the arrangement and
movement of particles in solids
The particles are packed closely together in a regular arrangement.
The particles vibrate in fixed positions.
Describe the arrangement and movement of particles in liquids
The particles are close together but able to move past each other.
The particles vibrate and move around each other.
Describe the arrangement and movement of particles in gases
The particles are well separated with no regular arrangement.
The particles vibrate and move freely at high speeds.
How do the relative energies of particles in a solid, liquid and gas compare?
Particles in a solid have the least amount of energy.
Particles in a gas have the most energy.
What are the names for the state changes from solid
to liquid and vice versa?
Solid → liquid: Melting
Liquid → solid: Freezing
Describe the forces between particles in solids, liquids and gases
Solids: Strong forces of attraction between particles which keep them in fixed positions.
Liquids: Weaker attractive forces than in solids.
Gases: Weakest intermolecular forces so particles move randomly.
How does a physical change differ from a chemical change?
A physical change involves changes in the forces between particles. The particles themselves remain the same and the chemical properties remain the same.
A chemical change is different as it affects the chemical properties of the substance.
Physical changes are relatively easy to reverse?
TRUE, Relatively easy to reverse since no new product is formed during the changes of state.
Describe what happens, in terms of particles, when a solid is heated and melts into a liquid
When heated, particles absorb thermal energy and convert it into kinetic energy.
The particles in the solid vibrate more, causing the solid to expand until the structure breaks and the solid turns into a liquid.
Describe what happens, in terms of particles, when a liquid is heated and evaporates into a gas
When heated, the particles in a liquid expand.
Some particles on the surface gain sufficient energy to overcome the intermolecular forces and evaporate.
At the boiling point, all of the liquid particles gain enough energy to evaporate.
Substance A melts at -200°C and boils at -183°C. What state is A at -174°C?
Gas
Substance B melts at -5°C and boils at 23°C. What state is A at -7°C?
Solid
Why do solids, liquids and gases expand when heated?
When a substance is heated the molecules vibrate faster.
This causes the space between the atoms to increase.
What is an advantage of the current particle model?
It provides a simple understandable model to explain the three states of matter.
Particles in the particle model are represented by ________ _______
Inelastic spheres
What are the limitations of the particle model?
- Doesn’t take into account forces of attraction between
particles. The amount of energy required to cause a
change of state depends on these forces.
- Doesn’t take into account the size of particles and space between them.
The nature of particles depends on the structure and bonding of a substance.
Liquid A has a higher boiling point than liquid B. What does this tell you about the forces between the particles in liquid A?
Liquid A has greater forces of attraction between the particles.
What is meant by the terms element and compound?
Elements are substances made up of only one type of atom.
Compounds are made up of atoms of different elements.
What are the three subatomic particles in an atom?
Protons
Neutrons
Electrons
Who described atoms as ‘solid spheres’?
John Dalton
What was Dalton’s theory?
- Atoms cannot be created, divided or destroyed.
- Atoms of the same element are exactly the same and atoms of different elements are different.
- Atoms join with other atoms to make new substances.
What discovery caused the Dalton model of an atom to change?
The discovery of subatomic particles
How did JJ Thomson discover the electron?
Thomson conducted an experiment using a cathode ray tube.
The beam moved towards the positively charged plate so he knew the particles must have a negative charge.
Describe the atomic model proposed by JJ Thomson
The plum pudding model:
Negative electrons scattered through a positively charged material.

Who designed and carried out the gold foil experiment?
Ernest Rutherford designed the experiment.
Geiger and Marsden carried out the experiment.

What did Rutherford discover from his gold foil experiment?
He shot a beam of positively charged particles into a sheet of gold foil.
- Most particles passed straight through indicating that atoms were mostly empty space.
- A few particles were deflected and a few bounced directly back showing that there must be a positively charged nucleus.
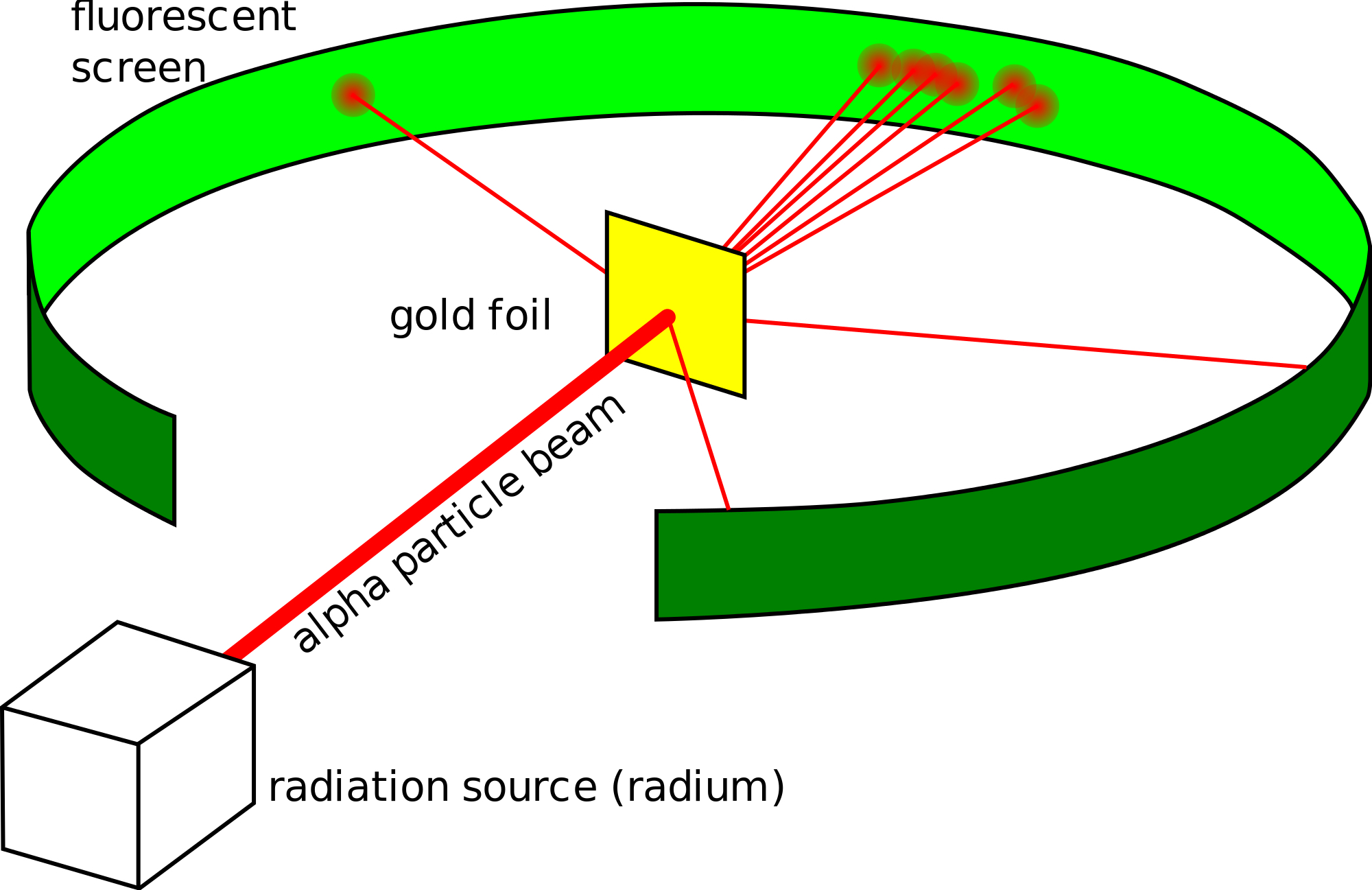
Describe Rutherford’s model of the atom
- Mass is concentrated in the central nucleus.
- Mostly empty space.
- Electrons travel in random paths around the nucleus.
What change did Niels Bohr propose to the nuclear model of an atom?
Electron shells around the nucleus.
Describe the structure of an atom
- A small central nucleus made up of protons and neutrons (positively charged).
- Electrons orbit (move around) the nucleus in shells (negatively charged).
Where is the mass of the atom concentrated?
In the nucleus
Compare the sizes of the nuclear radius and the atomic radius
The nuclear radius is much smaller than the atomic radius.
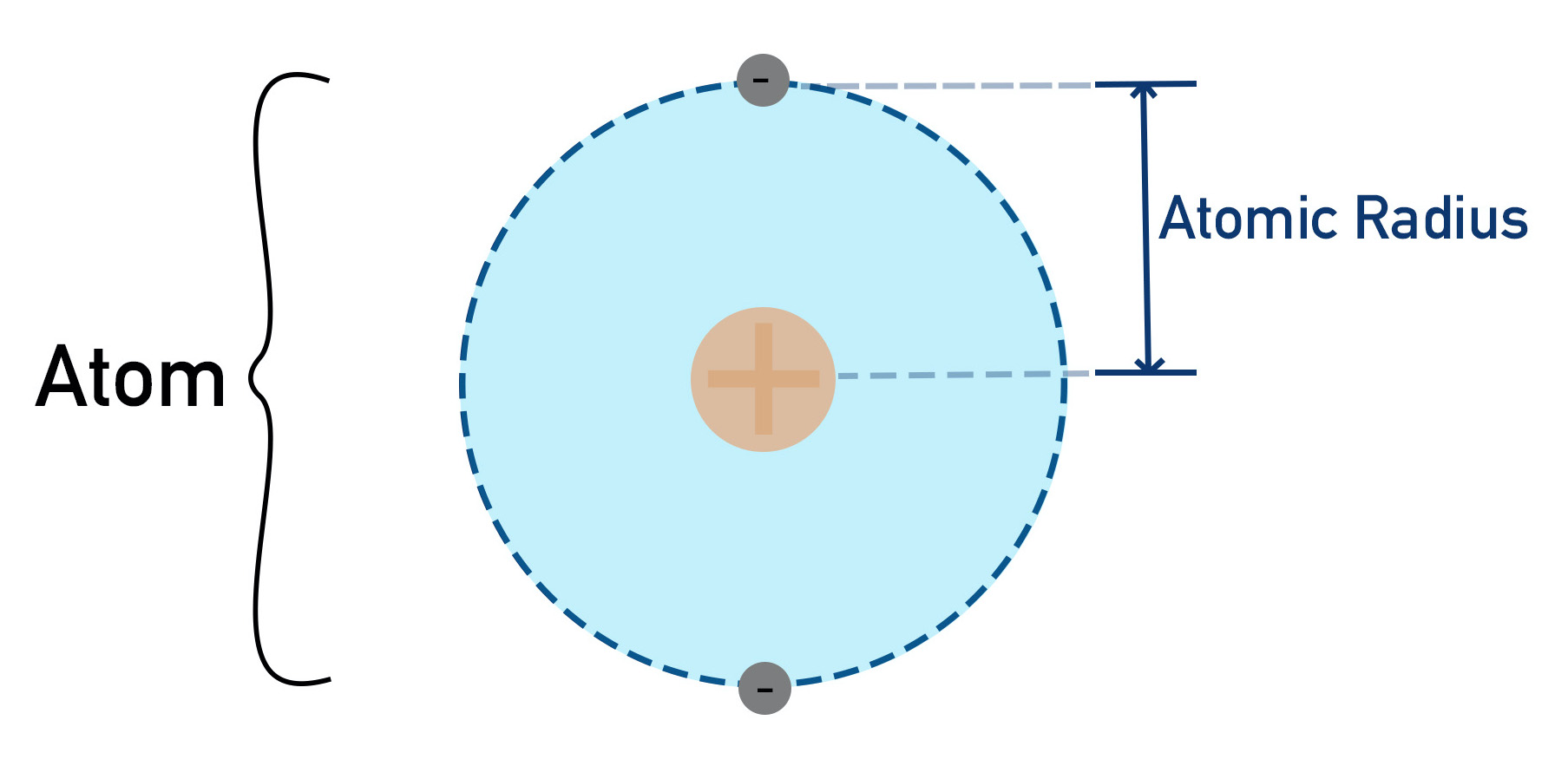
Compare the typical size of atoms and small molecules

What are the relative masses of a proton, neutron and electron?
Proton: 1
Neutron: 1
Electron: 1/1836
What are the relative charges of a proton, neutron and electron?
Proton: +1
Neutron: 0
Electron: -1
What is an ion?
An atom or molecule with a positive or negative charge.
How is an ion formed?
When an atom or molecule gains or loses electron(s).
Positive ions are formed when an electron is lost.
Negative ions are formed when an electron is gained.
What does the atomic number tell you about an element?
The atomic number is unique to each element and tells you the number of protons an element has.
What is the mass number?
The combined total of the number of protons and neutrons in the nucleus of an atom of an element.
What are isotopes?
Atoms of the same element with the same number of protons but a different number of neutrons.
How does the atomic number and mass number differ between isotopes of the same element?
Atomic number is the same as an element always has the same number of protons.
Mass number is different as there are different numbers of neutrons.
Why do atoms contain equal numbers of protons and electrons?
Atoms have a stable overall charge of 0.
Protons are positively charged and electrons arenegatively charged so they must be present in equal numbers for charges to balance.
How can you calculate the number of neutrons, given the mass number and atomic number of an element?
Number of neutrons = mass number - atomic number
Boron has the atomic number 5 and mass number 11. How many protons, electrons and neutrons does boron have?
5 protons
5 electrons
6 neutrons
Sodium has the atomic number 11 and mass number 23. How many protons, electrons and neutrons does the Na + ion have?
11 protons
10 electrons (one has been to form the positive ion)
12 neutrons