Honors General Chemistry: Midterm 1 (Chapters 1-3)
1/83
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
84 Terms
1012
Tera
109
Giga
106
Mega
103
Kilo
102
Hecto
101
Deca
10-1
Deci
10-2
centi
10-3
milli
10-6
micro
10-9
nano
10-12
pico
10-15
femento
4.00 × 10-6
What is the best way to represent 0.00000400g in scientific notation?
We use significant figures for precision since there is some uncertainty in the reported digit.
Why do we use significant figures to express most of our answers?
6.022 × 1023
What is Avogrado’s Number?
They are inverses.
What is the relationship between wavelength and energy (or frequency)
3.00×108
What is the constant speed for wavelength and frequency?
c/v
What is the wavelength formula?
The particle of light.
What does the wavelength measure?
When they surpass the threshold frequency.
How can electrons/photons be photoejected?
This is through increasing the light intensity
How can more photons/electrons be photoejected?
Photons
Massless particles of electromagnetic radiation.
Ephoton
What is known as the energy of a single photon of light that has a frequency & a wavelength?
hc/λ
What is the formula for Ephoton ?
6.626×10-34
What is Plank’s Constant?
It is the amount of energy required to liberate an electron from that material’s surface.
What does the binding energy do?
KE = ½ mv2 and KE = Ephoton -W
What are the 2 formulas for Kinetic Energy?
9.11×10-31
What is the mass of an electron?
deBrogile wavelength
What is the wavelike behavior of matter known as?
The particles of light are massless.
Why doesn’t deBrogile wavelength measure particles of light?
Emission
Energy going from a higher to lower energy state is known as..
-2.179 × 10-18
What is the ionization energy of a Hydrogen atom?
n
The principal quantum number
l
The angular momentum
ml
The magnetic quantum number
-Z2 B(1/n2f - 1/n2f )
What is the formula of a transition/change between energy states?
| -Z2 B(1/n2f - 1/n2f ) |
What is the formula of the Ephoton?
n-l-1
How can the number of radial nodes be found?
kilograms
What are the units of mass that should be used when finding the KE & deBrogile wavelength?
1.602 × 10-19
1eV equals to how many Joules?
ns
If you try to distinguish a ns orbital and a np orbital, which one would have the stronger pull towards the nucleus?
d orbital
Would the p orbital or the d orbital have more shielding?
This is because of shielding and electron-electron repulsion causing the electrons to feel the pull of the nucleus.
Why don’t electrons in multi-electron species experience the full nuclear charge?
This is due to electron-electron repulsion & shielding.
Why is the effective nuclear charge of Helium is always less than 2?
Core Electrons
These type of electrons experience a relatively high effective nuclear charge & are low in energy.
Valence Electrons
These electrons experience a low effective nuclear charge and are high in energy.
Z (atomic number) - O (core electrons)
How can the estimation of the nuclear charge be calculated?
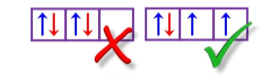
Pauli Exclusion Principle
This rule states that no two electrons in the same species can have all four quantum spin numbers be equivalent
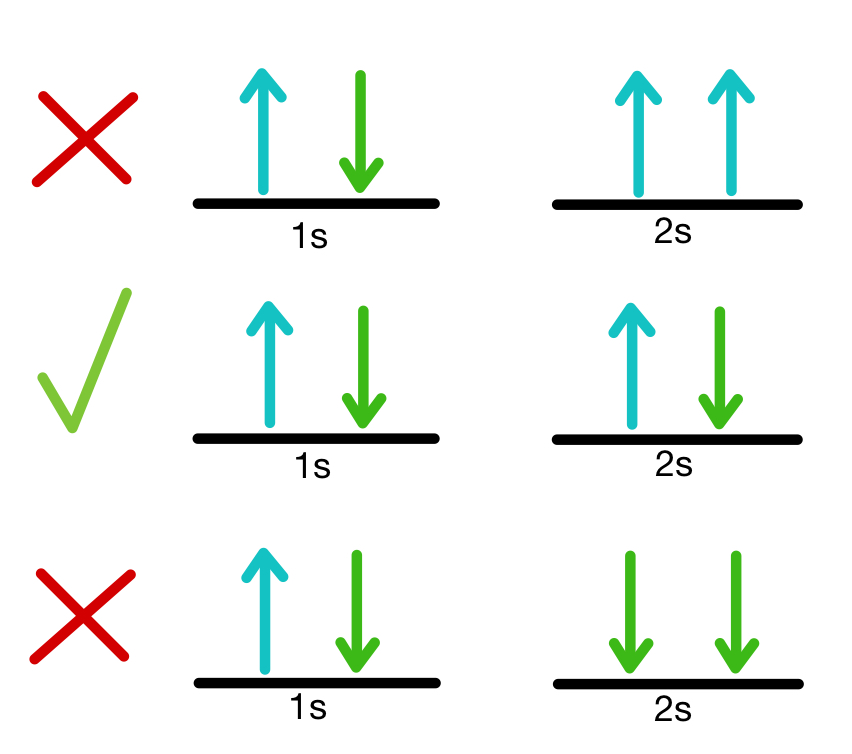
Hund’s Rule
This rule states that the lowest energy configuration maximizes total spin-meaning that we don’t pair electrons unless needed.
An s orbital.
Would an s or a d orbital have higher penetration ability?
Aufbau Principle
This principle states that we have to start by filling up the lowest energy orbital first. (going from s, p, to d)
s orbital due to being farther away from the nucleus.
If we were to remove an electron because of an cation, would we remove it from the s or the d orbital?
[Ar] 4s1 3d5
What is the electron configuration for Chronium?
[Ar] 4s1 3d10
What is the electron configuration for Copper?
[Kr] 5s1 4d5
What is the electron configuration for Molybdenum?
[Kr] 5s1 4d10
What is the electron configuration for Silver?
[Xe] 6s1 4f14 5d10
What is the electron configuration for Gold?
When one or more electrons are in higher energy levels than in ground state.
Example: 1s1 2s1
What is an excited state?
Paramagnetic
Species with one or more unpaired electrons that are attracted to an external magnetic field is known as..
Diamagnetic Species
Species with electrons that are all paired that are slightly repelled by an external magnetic field is known as..
Effective Nuclear Charge
This periodic trend increases across a period because more valence electrons are being added to a given subshell - protons are being added & there are no core electrons being added to increase the shielding.
The core electrons began to increase in those elements.
Why doesn’t Lithium and Potassium have a high nuclear charge despite having more protons?
Atomic Radius
This periodic trend increases due to the number of core electrons being added, causing more shielding. Also the increase of electron-electron repulsion causes the increase
Because electrons are being removed from their neutral species, it decreases electron-electron repulsion.
Why do cations have a small atomic radii?
Because electrons are being added, this increases electron-electron repulsion.
Why do anions have a larger radi?
We look for the number of protons in the element and whichever has the most protons has the smallest atomic radius, but whichever has the smallest protons has the largest atomic radius. The element having the most positive charge would have the smallest atomic radius.
What do we look for in isoelectronic species when we are sorting out atomic radius?
The amount of energy required to remove a mole of its outermost valence electrons from a mole of gas-phase species.
What is ionization energy?
This is due to the increasing effective nuclear charge - meaning the electrons will be more bounded tightly to the nucleus, making it harder to remove the electrons from the atom.
Why does IE1 increase across?
The p subshell is being populated, causing it to take more energy to remove compared to the s subshell.
Why is there an anomaly in the 2A and the 3A group (only in Be & B/Mg & Al)
This is due to the electron pairing causing electron-electron repulsion, which needs more energy to be removed.
Why is there an anomaly in 5A & 6A group?
The number of protons should be looked upon, and it shows that a large amount of energy is needed to liberate the electron.
In an isoelectronic species, what should we be aware of when we find the one with the largest IE1?
This is when we start to remove core electrons which needs more energy to remove.
Why does IE have a large gap as we go through some of the trends?
Electron Affinity
The amount of energy released when one mole of electron is added to one mole of that species in the gas phase.
This is when the 3s subshell is resistant to give up or pair with an electron. - also the elements for group 8A are already stable, so they wouldn’t accept more electrons
Why is there an anomaly for the Electron Affinity for Group 8A?
This is because electrons would be added to a new shell.
Why does Group 8A have positive IE?
Energy is increasing
What happens to energy when energy states are being absorbed?
Energy decreases
What happens to energy when energy states are being emitted?
X+(g) → X2+(g) + e-
How can the second ionization energy be described in an equation?
X(g) → X+(g) + e-
What is the ionization energy formula?
X(g) + e- → X-(g)
What is the electron affinity formula?
C. GS < ES-B < ES-A
The Green Fluorescent Protein (GFP), and its derivatives, are commonly used as biological markers in modern molecular biology experiments.
The protein can absorb light at 395 nm, which transitions the protein from its ground electronic state (GS) to an excited electronic state (ES-A).
The protein then transitions to another state (ES-B) before, finally, emitting light with a wavelength of 510 nm, which we see as green. Based on the information above, what can be concluded about the ordering of energy of GS, ES-A and ES-B?
A. GS < ES-A < ES-B
B. GS = ES-B < ES-A
C. GS < ES-B < ES-A
D. GS = ES-A = ES-B
A. The extent (size) of the orbitals in the valence shell increases down a group, decreasing how tightly bound the valence electrons are bound to the nucleus and decreasing the energy required to remove the outermost electron.
9. Consider the following observed trend in first ionization energies of some of the alkaline earth metals: Sr < Ca < Mg Select the best explanation for this trend.
A. The extent (size) of the orbitals in the valence shell increases down a group, decreasing how tightly bound the valence electrons are bound to the nucleus and decreasing the energy required to remove the outermost electron.
B. The number of protons increases down a group, increasing the atomic radius and decreasing energy required to remove the outermost electron.
C. The number of valence electrons increases down a group, decreasing the energy required to remove the outermost electron.
D. The size of the nucleus increases down a group, increasing the atomic radius and decreasing the energy required to remove the outermost electron
B. n = 3, ℓ = 2, mℓ = +2, ms = +1/2
11. Which of the following combinations of quantum numbers could specify a valence electron in Zn?
A. n = 4, ℓ = 0, mℓ = -1, ms = -1/2
B. n = 3, ℓ = 2, mℓ = +2, ms = +1/2
C. n = 3, ℓ = 1, mℓ = +1, ms = -1/2
D. n = 2, ℓ = 2, mℓ = -2, ms = +1/2