Ceramic Unit Cells
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
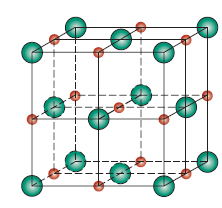
Zinc Blende (ZnS)
- n’ = 4
- C = 4
- r-ratio: (0.225-0.414)
- cation location: tetrahedral
- structure: FCC
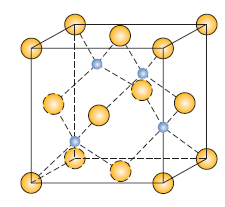
Rock Salt (NaCl)
- n’ = 4
- C = 6
- r-ratio: (0.414-0.732)
- cation location: octrahedral
- structure: FCC
- a = 2r+2r
- 1 anion : 1 cation
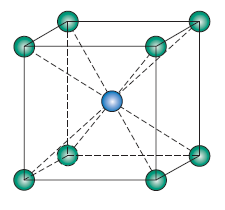
Cesium Chloride (CsCl)
- n’ =
- C = 8
- r-ratio: (0.732-1.0)
- cation location: cubic
- structure: cubic
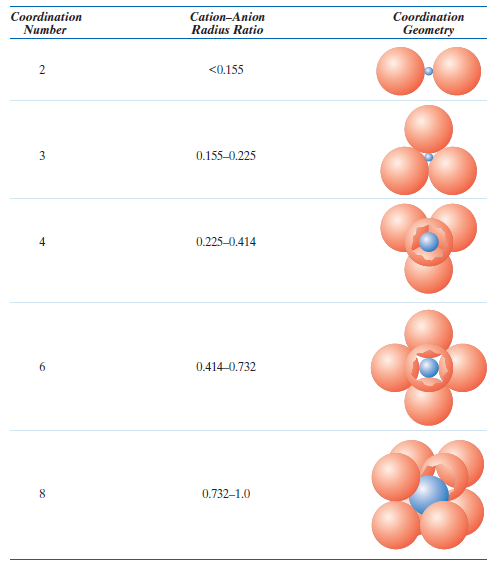
Chart
- Linear
- Triangular
- Tetrahedral → Zinc Blende
- Octahedral → Rock Salt
- Cubic → Cesium Chloride
AmXp (Flourite CaF2)
- n’ = 4
- C(cation) = 8
- C(anion) = 4
- r-ratio: (0.225-0.414)
- cation location: cubic
- 1 anion : 2 cation
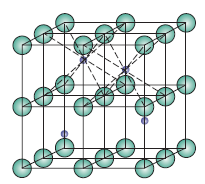
AmBnXp (Perovskite)
- n’ = 4
- C(cation) = 12
- C(anion) = 6
- r-ratio: (0.225-0.414)
- cation location: FCC
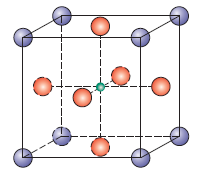
Diamond
Same as zinc but just all diamond
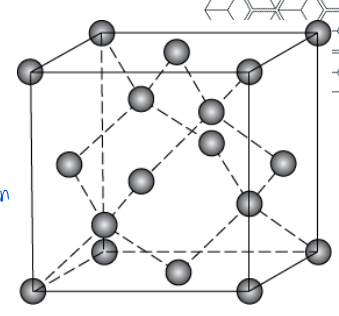
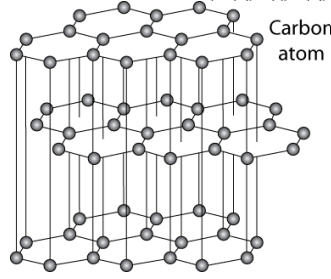
Graphite
layered structure of graphene layers with van der waals forces between them