Topic 15: Metal complexes (ion)
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
What is the definition for metal complex/metal complex ion?
When a central transition metal ion is surrounded by ligands which are bonded by dative covalent bonds
(If there is an overall charge then it is a metal complex ion)
What is the definition of ligands?
Species that have 1 or more lone pairs (they are electron-rich), they can be monodentate, bidentate or polydentate
What type of bonds do ligands form?
Dative bonds
What are monodentate ligands?
Ligands which can only form one dative bond with another species (only have one lone pair)
What are examples of monodentate ligands?
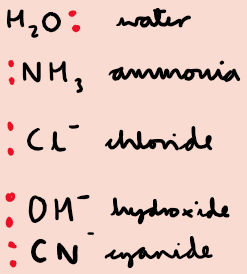
What are bidentate ligands?
Ligands which can form 2 dative bonds (have two lone pairs)
What are examples of bidentate ligands?
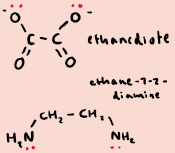
What are polydentate ligands?
Ligands which can have more than 2 coordinate bonds
What are examples of polydentate ligands?

What are examples of small ligands?
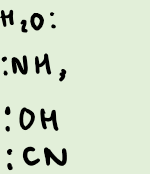
How many small ligands can you fit around the central atom?
6
What are examples of larger ligands?
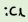
How many larger ligands can you fit around a central atom?
4
What is an example of LARGEEE ligand?
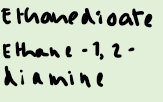
How many LARGEEE ligands can you fit around a central atom?
3 (normally)
What is a coordination number?
Number of coordinate bonds NOT number of ligands
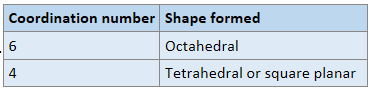
When a complex ion has a coordiantion number of 6 and 4, what are their shapes respectively?
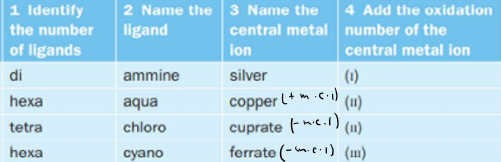
How do you work out the overall charge of a metal complex?
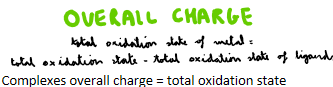

Naming thing