Oxidation Reactions and Reactants
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
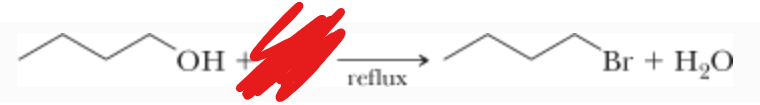
What is the reactant?
What is the mechanism?
HBr —>
SN2
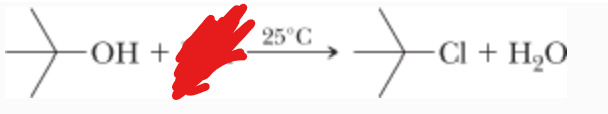
What is the reactant?
What is the mechanism?
HCl —>
SN1
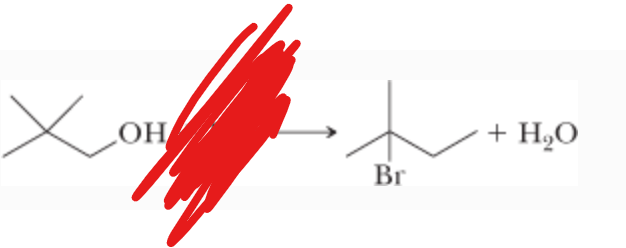
What is the reactant?
What is the mechanism?
(Works well with tertiary carbons)
HBr —>
SN1
What is the reactant?
(Works well with primary and secondary carbons)
Inversion!!!
PBr3 —> CH2Cl2
What is the reactant?
(Works well with primary and secondary carbons)
Inversion!!!
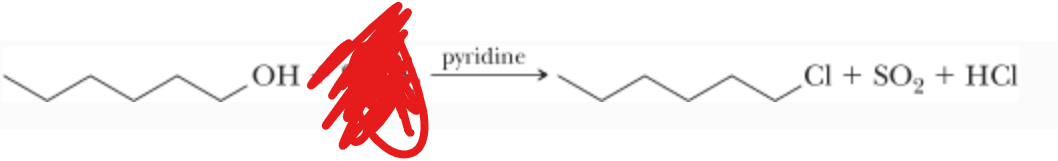
SOCl2 —> pyridine
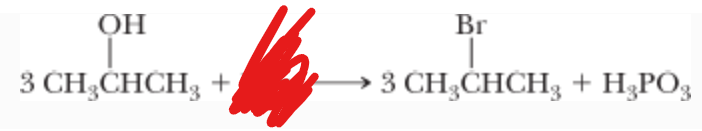
What is the reactant?
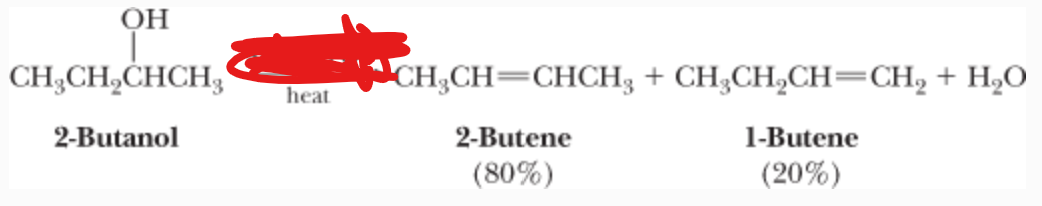
H3PO4 —> heat
What is the reactant?
Removes OH and converts the other OH to double bonded O
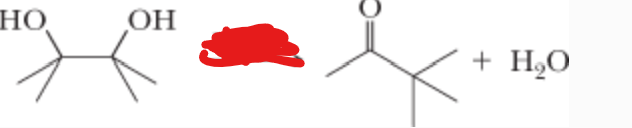
H2SO4 —> H2O
What is the reactant?
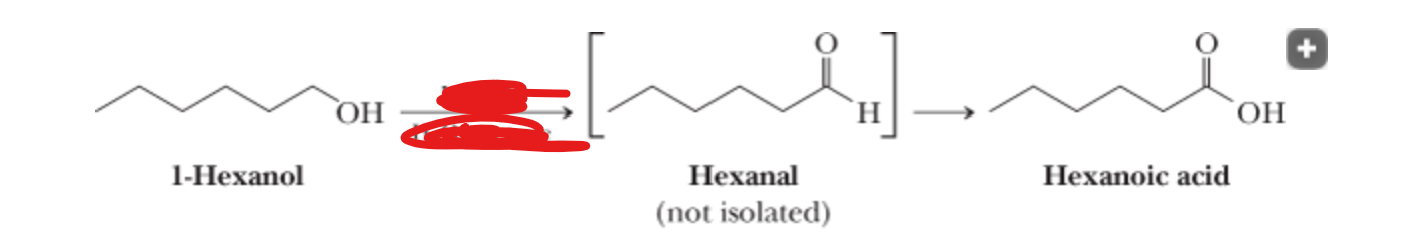
H2CrO4 —> H2O, Acetone
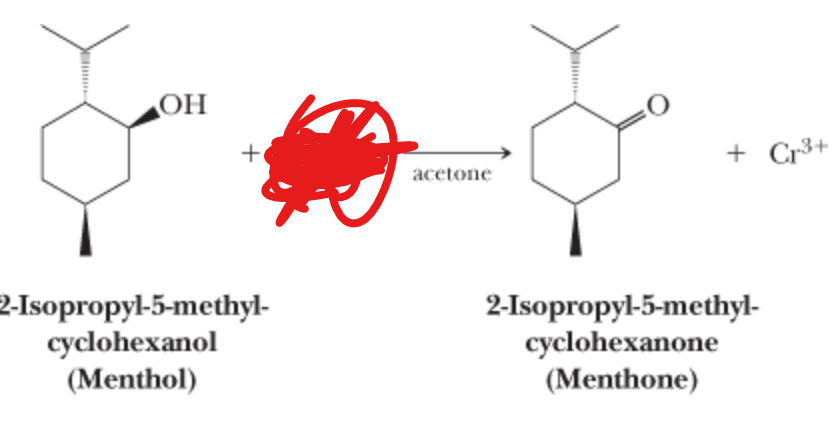
What is the reactant?
H2CrO4 —> Acetone
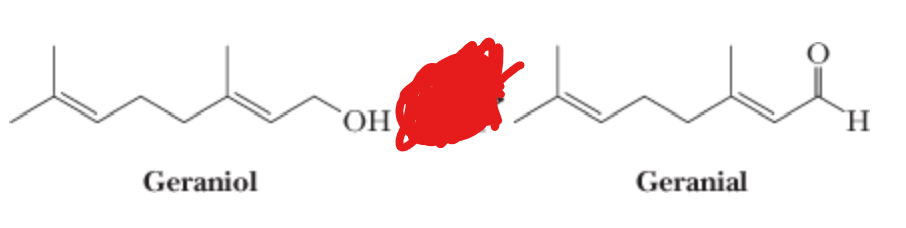
What is the reactant?
PCC —> CH2Cl2
What reactants can be used to oxidize secondary alcohols into ketones?
H2CrO4 —> Acetone
PCC —> CH2Cl2
1) COCl2, DMSO —> 2) Et3N
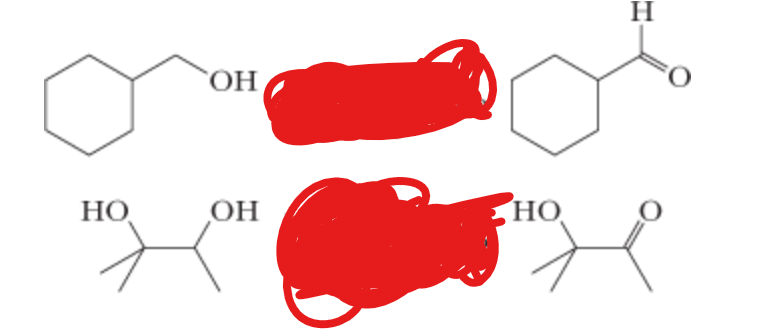
What is the reactant?
1) (COCl)2, DMSO —> 2) Et3N
What is the reactant?
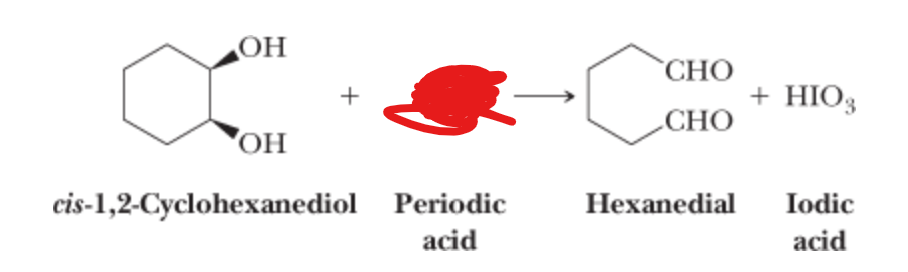
HIO4 —>
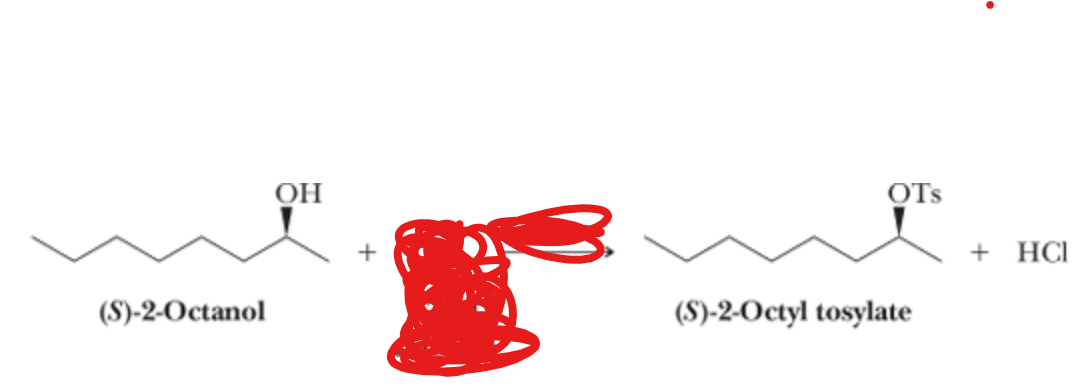
What is the reactant?
TsCl —> pyridine
Oxidizing secondary alcohols into ketones and primary alcohols into carboxylic acids (2):
K2Cr2O7 —> H2SO4 H2O
CrO3 —> H2SO4 H2O
Reduces aldehydes into primary alcohols and ketones into secondary alcohols
NaBH4 —> CH3OH