Water [IB Biology HL]
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
53 Terms
In water, covalent bonds are found between...
hydrogen and oxygen atoms.
Hydrogen bonding occurs when...
partially positive hydrogen atoms of one polar molecule are attracted to the partially negative atoms of another polar molecule.
covalent
a bond formed by sharing electrons.
polar
molecule or bond with an uneven distribution of charge.
nonpolar
molecule or bond with an equal distribution of charge
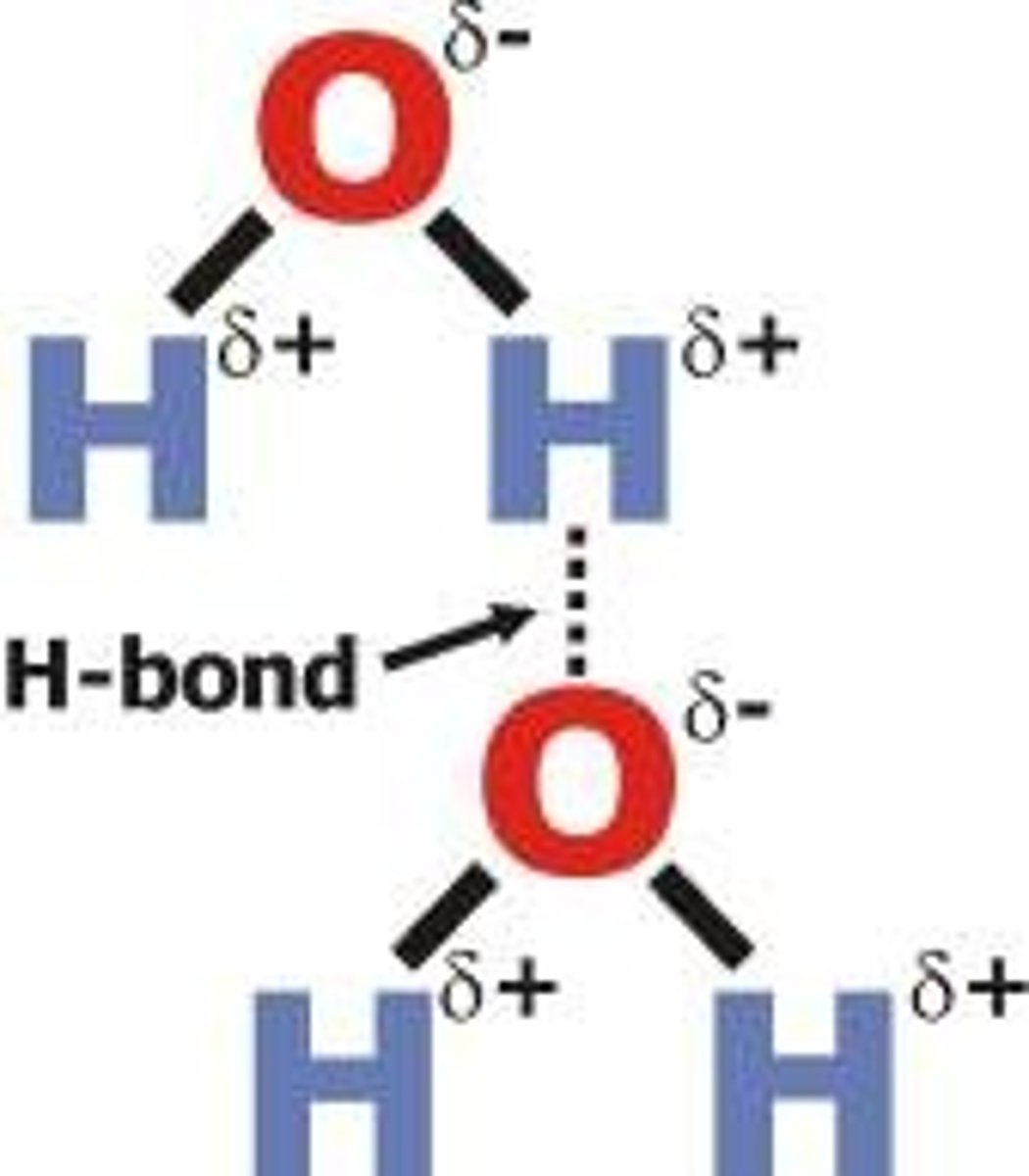
hydrogen bond
a weak attraction between a hydrogen atom of one polar molecule and an electronegative atom of another polar molecule.
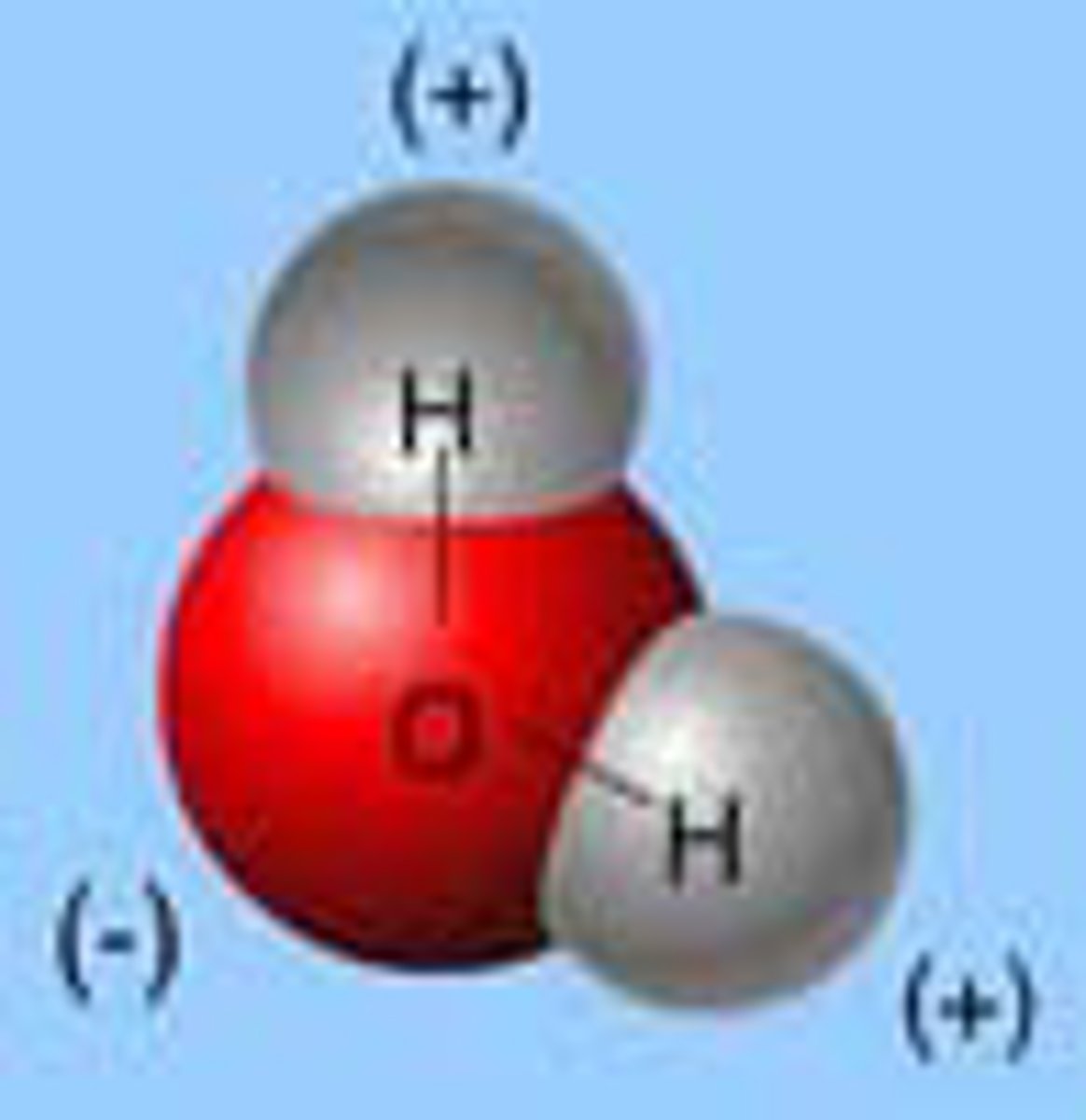
A water molecule is composed of...
two hydrogen atoms, each bonded to an oxygen atom.
A covalent bond is a type of bond formed when...
atoms share electrons.
In a water molecule, the oxygen and hydrogens...
share their electrons.
Because oxygen is more electronegative than hydrogen...
oxygen pulls the shared electrons in the covalent bonds closer to itself. This unequal distribution of electrons results in the oxygen atom having a partial negative charge while the hydrogen atoms have a partial positive charge.
An atom's charge is represented by the Greek lowercase symbol...
delta.
The water molecule has a bent shape with an angle of approximately ___ degrees between the hydrogen-oxygen-hyrdrogen bonds.
105
The water molecule's bent shape combined with its uneven electron distribution (polarity) creates an overall...
dipole.
Since water is polar, water molecules are...
attracted to each other through the force of hydrogen bonding.
Hydrogen bonds form between...
a negative oxygen atom of one water molecule and a positive hydrogen atom of another molecule.
Hydrogen bonds are...
strong intermolecular forces.
Water's high boiling point and role as a versatile solvent can be explained by...
hydrogen bonds as strong intermolecular forces.
Because water is polar, other polar molecules are attracted to it, so...
hydrophillic substances can dissolve in water as they form hydrogen bonds with the water molecules.
It is believed that, in Earth's primordial oceans 3.8 byo, critical compunds came together to form...
polymers needed to build cells. Water was the perfect medium for this because the enzymes required to catalyze these reactions function in aquous environments and the molecules can move freely in water to collide with one another and start the reactions. We still see this in the cytosol of modern-day cells.
The cytosol is...
the fluid encased by the cell membrane. It is composed of around 70 percent water and is the site of most chemical reactions conducted by the cell
The solvent ability of water is complemented by the fact that...
hydrophobic substances cannot dissolve into water; instead they repel water, giving rise to new properties. For instance, lipids are hydrophobic and insoluble cholesterol is an example of a key lipid that's required for cells as it affects the fluidity of the cell membrane.
Because of its insolubility, cholesterol can be...
embedded into the equally hydrophobic core of the plasma membrane instead of being repelled. However, when it comes to transport, cholesterol needs to be packaged into lipoproteins like LDL and HDL cholesterols.
As a hydrophobic molecule, cholesterol won't dissolve into...
blood. The main component of blood is plasma, which is about 90 percent water. The plasma can dissolve hydrophillic molecules for transport but requires hydrophobic molecules to be repackaged into a hydrophillic container.
Aquatic habitats like marine environments benefit from several of water's physical properties. For example, in the habitat of the Arctic sea, the ringed seal lives within the water, whereas the black throated loon lives in the coastal environment. Four physical properties of water that impact this habitat include...
specific heat capacity, thermal conductivity, buoyancy, and viscosity.
The specific heat capacity of a substance is...
the amount of (heat) energy required to change the temperature of one unit mass of the substance by one degree celsius (or one Kelvin).
The practical effect of water's specific heat capacity is that...
it reflects the amount of energy a substance can absorb.
Water has a ________ specific heat capacity.
high
Water can absorb a significant amount of heat from the surrounding environment, and then release it slowly as the environmental temperature decreases.
Specific heat in arctic habitats
Over the warmer summer months, heat from the sun and the air is absorbed by the ocean, stored in the water by breaking the hydrogen bonds that hold it together. Then, as the temperature cools in the winter, the hydrogen bonds reform slowly and release that energy and heat over time. This coolant property allows bodies of water to create warmer microclimates, as it buffers the surrounding area from the cold. This allows the ringed seal and the black throated loon to live comfortably.
Thermal conductivity is...
the ability of a substance to conduct or transfer heat; how readily heat flows through a material when a temperature difference is present. Higher values indicate greater heat conduction.
Relative to air, water has a ____ thermal conductivity.
high
Thermal conductivity in arctic habitats
In water, the strong hydrogen bonding network facilitates rapid energy transfer, leading to higher thermal conductivity. Heat transfers into and out of water quickly, so as a warm-blooded mammal, the ringed seal needs to insulate itself well against heat loss while swimming, resulting in its thick layer of blubber. Air is primarily composed of non-polar molecules like nitrogen and oxygen, which have weak intermolecular forces. Heat transfer in air occurs through collisions between relatively few and weakly interacting gas molecules, resulting in lower thermal conductivity. The black throated loon, living predominantly in the land and air, does not lose as much heat to its environment relative to the seal.
Buoyancy is...
the upward force exerted by a fluid, which acts against the downward force of gravity.
Buoyancy is determined by...
the density of a medium.
Buoyancy in arctic habitats
Water is more dense than air, so it has a comparatively greater buoyancy. This upward force allows objects and organisms to float in the ocean. Therefore, the ringed seal can swim more easily and spend less energy on keeping itself afloat. Air has no buoyancy, so the black throated loon expends more energy keeping itself lifted in the air compared to the ringed seal in the water. The black throated loon has to overcome the force of gravity during flight, whereas the ringed seal is powered by the force of buoyancy.
Viscosity is...
the resistance of a liquid to flow. As a liquid, water is viscous where air is not.
Viscosity in arctic habitats
The seal swimming is more efficient because of water's viscosity. Water provides resistance when the ringed seal's flippers push against it, propelling the seal forward. When the loon uses its webbed feet to swim, it uses the same viscosity to its advantage. Relative to other liquids, however, water has a low viscosity, which means significant energy is not needed for organisms to move through water.
Hydrogen bonds form between the positive hydrogen atoms and the negative oxygen atoms of two different molecules. This results in polar molecules having different forces of attraction, giving rise to a phenomenon called...
cohesion.
Cohesion occurs when...
molecules of the same polar substance are attracted to one another.
As a consequence of cohesion and because water molecules link using hydrogen bonds...
surface tension occurs.
Surface tension is...
the elasticity of a liquid surface.
The surface tension of water can...
resist external forces like a light insect moving across it or a paper clip being rested on top. Small objects can distribute their weight equally over the surface of the water, moving across without breaking the hydrogen bonds.
Water's hydrogen bonds contain energy that...
produce its cohesive force, because it takes a greater amount of energy to break the bonds and separate the water molecules.
The cohesion and surface tension of water create...
thriving habitats on water surfaces, composed of insects, algae, frog eggs, water spiders, and many other organisms.
Another force observable in polar molecules is adhesion, which is...
the tendency of (water) molecules to stick to other substances rather than each other. This attraction can be greater than, equal to, or less than the cohesive force.
Adhesion also plays an important role in biological processes. For example, in the xylem of plants...
adhesion is used to transport absorbed water from the roots upwards. Xylem are a type of vascular tissue -- long, thin tubes that extend from the roots up through the stem and into the leaves. The walls of the xylem are plant cells walls composed primarily of cellulose. Cellulose has a number of hydroxyl groups that readily form hydrogen bonds with water molecules. This attraction causes water to stick to the xylem walls. Cellulose is hydrophilic, so even though water is moving up the xylem against the force of gravity, this adhesion helps prevent it from falling down. This force of adhesion is complemented by cohesive forces, resulting in a phenomenon called capillary action. Capillary action is when a liquid like water moves upwards in a thin tube against the force of gravity. Cohesion plays a role in this process because the water molecules are attracted to each other; when one molecule is pulled upwards, the next molecule follows, and so forth. This is often called the beads on a string model because the negative pressure at the top of the xylem (caused by evaporation) pulls the water molecules upwards like beads on a string. Adhesion causes the water molecules to stick to the xylem walls before cohesion causes them to follow each other, eventually sticking to the wall higher up the tube. This repeats until the water exits the xylem into the leaves, where it evaporates through pores called stomata and moves out into the atmosphere. This is also called the cohesion tension theory, and it is the mechanism behind transpiration in plants.
Capillary action in soil is a critical process also influenced by...
the adhesive properties of water.
In the context of soil, adhesion refers to the...
attraction between water molecules and the surfaces of soil particles.
Soil particles , which can be composed of sand, silt, clay, and organic matter, have various surface properties that can...
attract and hold water molecules.
The adhesive forces between the water molecules and the soil particles are responsible for the...
initial wetting of the soil.
Another way to describe capillary action is the movement of...
water within the spaces of a porous material due to the forces of adhesion, cohesion, and surface tension.
When water is present in the soil, it moves through the tiny spaces between soil particles due to the forces of adhesion, cohesion, and surface tension. We can think of these spaces as capillaries like the tubes of the xylem. Adhesion causes the water to cling to the soil particles, while cohesion helps pull more water molecules along with it. The finer the soil particles, the...
smaller the capillary spaces, which enhances capillary action.
Surface tension contributes to the soil's ability to retain water by...
holding it in the porous spaces against the force of gravity.
The combination of capillary action and surface tension allows water to...
move upward against gravity in the soil, ensuring that water can reach plant roots even when the water table is lower than the root area. This maintains the soil moisture, which is vital for seed germination, root development, plant hydration, and nutrient uptake.