Calorimetry: Energy Conservation, Specific Heat, and Temperature Calculations
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
What is the main concept behind calorimetry?
Conservation of energy, where energy lost by a higher temperature substance is gained by a lower temperature substance.
What is the formula used to calculate energy change in calorimetry?
Q = mcΔT, where Q is the heat energy, m is the mass, c is the specific heat capacity, and ΔT is the change in temperature.
What is the specific heat capacity of water?
4.186 J g⁻¹ °C⁻¹
How do you express the equality of energy changes between two substances in calorimetry?
mHcHΔTH = mLcLΔTL, where H is the higher temperature substance and L is the lower temperature substance.
What is the increase in internal energy of a 63 g piece of lead heated from 22 °C to 110 °C with a specific heat capacity of 128 J kg⁻¹ K⁻¹?
710 J
What is the mass of a piece of copper if its internal energy decreases by 220 J when it goes from 86 °C to 14 °C with a specific heat capacity of 0.386 J g⁻¹ K⁻¹?
7.9 g
What is the final equilibrium temperature when mixing 45.0 g of water at 20.0 °C with 65.0 g of water at 30.0 °C?
25.9 °C
What is the final equilibrium temperature when mixing 90.0 g of water at 20.0 °C with 200.0 g of water at 40.0 °C?
33.8 °C
What is the specific heat of aluminum if a 28.4 g sample heated to 39.4 °C is placed in 50.0 g of water, raising the water's temperature from 21.00 °C to 23.00 °C?
0.901 J g⁻¹ °C⁻¹
How do you calculate the specific heat of a metal piece weighing 59.05 g heated to 100.0 °C and placed in 96.5 mL of water at 23.7 °C, reaching an equilibrium temperature of 27.8 °C?
0.388 J g⁻¹ °C⁻¹
What is the importance of isolating the system in calorimetry experiments?
To keep all energy contained within the system for accurate measurements.
What happens to the energy of the higher temperature substance in a calorimetry experiment?
It is transferred to the lower temperature substance, causing the latter's temperature to increase.
What are the common units used in calorimetry for mass and temperature?
Grams (g) for mass and degrees Celsius (°C) for temperature.
What is the significance of assuming no phase changes in calorimetry calculations?
It simplifies calculations by allowing the use of Q = mcΔT without accounting for latent heat.
In calorimetry, what does ΔT represent?
The change in temperature of the substance, calculated as final temperature minus initial temperature.
Why is it important to show all work and include units in calorimetry problems?
To ensure accuracy and clarity in calculations, making it easier to identify errors.
What is the relationship between mass and specific heat in calorimetry?
The mass of a substance multiplied by its specific heat capacity determines how much energy it can absorb or release during temperature changes.
What is the purpose of using a calorimeter in experiments?
To measure the heat transfer during physical or chemical processes.
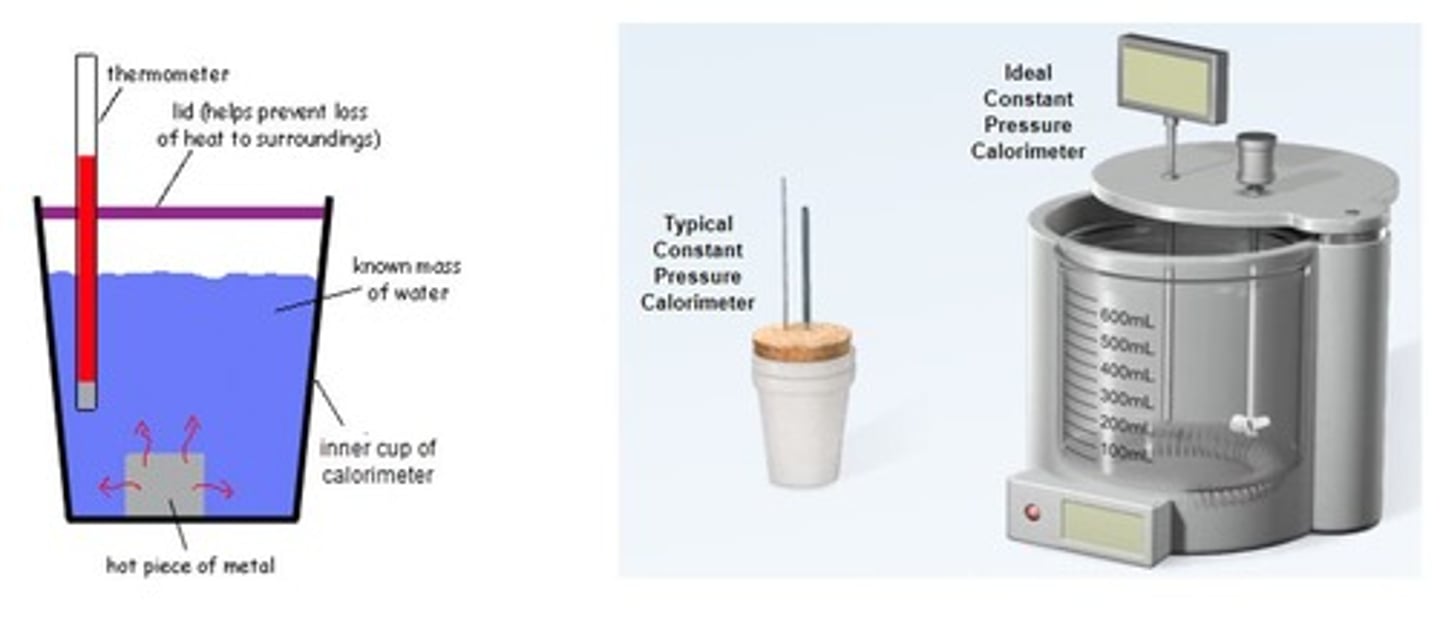
What does it mean when two substances reach thermal equilibrium in calorimetry?
They have reached the same temperature, and there is no net heat transfer between them.