entropy & feasibility
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
entropy definition
quantitative measure of disorder in a system (JK^-1mol^-1) more disorder higher entropy higher temp
standard entropy definition
entropy content of 1mol of substance under standard conditions
is entropy always +ve or always -ve
always +ve as all substances possess a degree of disorder
what is the type of entropy from solid to liquid
entropy of fusion
what is the entropy liquid to gas
entropy of vaporisation , (greater than entropy of fusion)
Total entropy equation
S(total)= S(products)- S(reactants)
all spontaneous reactions have ____ entropy
+ve
at 0K perfect crystals have
0 entropy
free energy change equation
G= H-TS

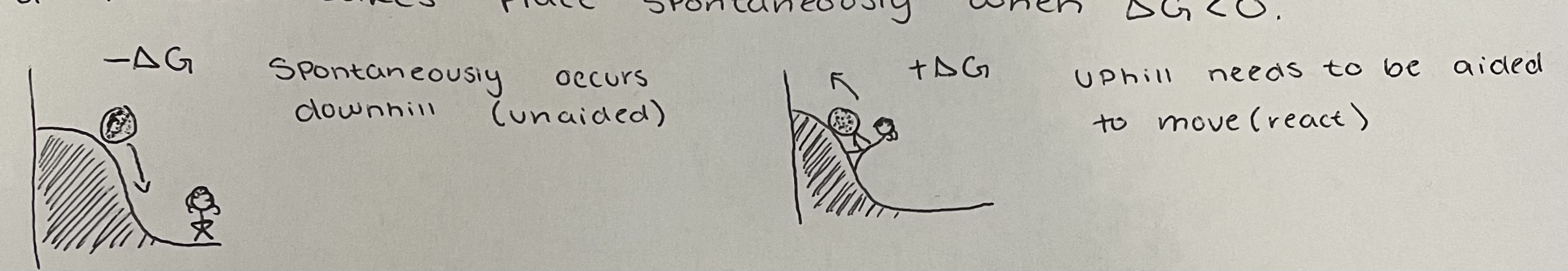
a process takes place spontaneously/ is feasible when
G<0
visual for gibbs
Gibs feasibility table

when finding T
set G=0
reactions occurring depends on
• activation energy
• slow rate of reaction