Hess's Law
0.0(0)
Card Sorting
1/4
There's no tags or description
Looks like no tags are added yet.
Last updated 6:51 PM on 8/7/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
1
New cards
first law of thermodynamics
energy cannot be created or destroyed
2
New cards
Hess’s law
total enthalpy change in a chemical reaction is independent of the route by which the chemical reaction takes place as long as the initial and final conditions are the same
3
New cards
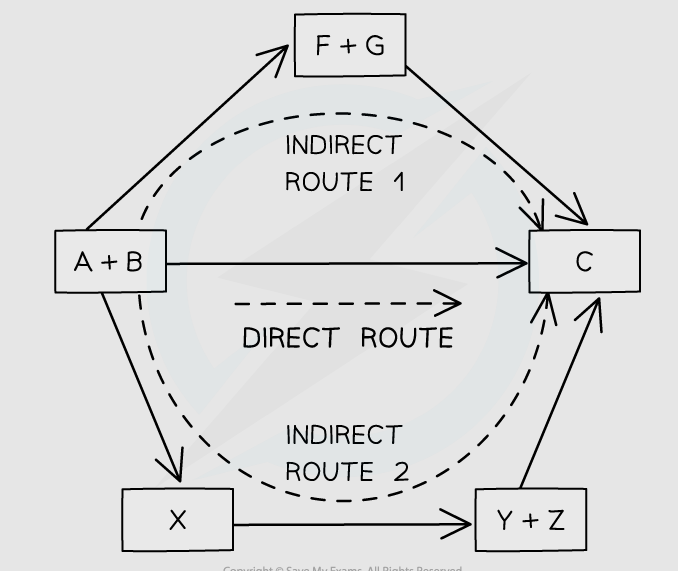
energy cycles
enthalpy change of direct route is the same as the enthalpy change of the indirect routes
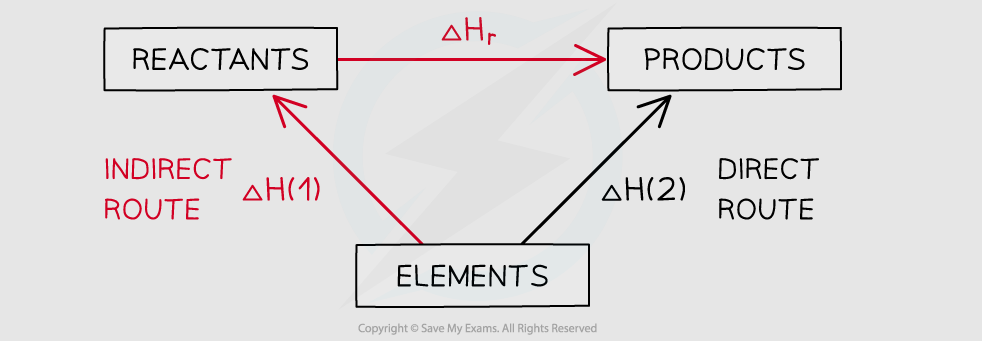
4
New cards
energy cycle for enthalpy change of reaction
Hr=H2-H1…write balanced equation at top…elements at bottom
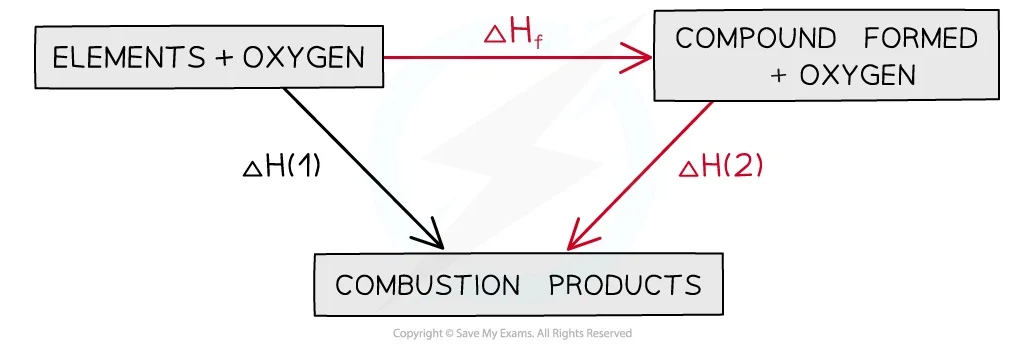
5
New cards
energy cycle for enthalpy change of combustion
Hf=H1-H2…write equation on top adding oxygen to both reactants and products…water and oxygen at bottom