ionic bonding
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
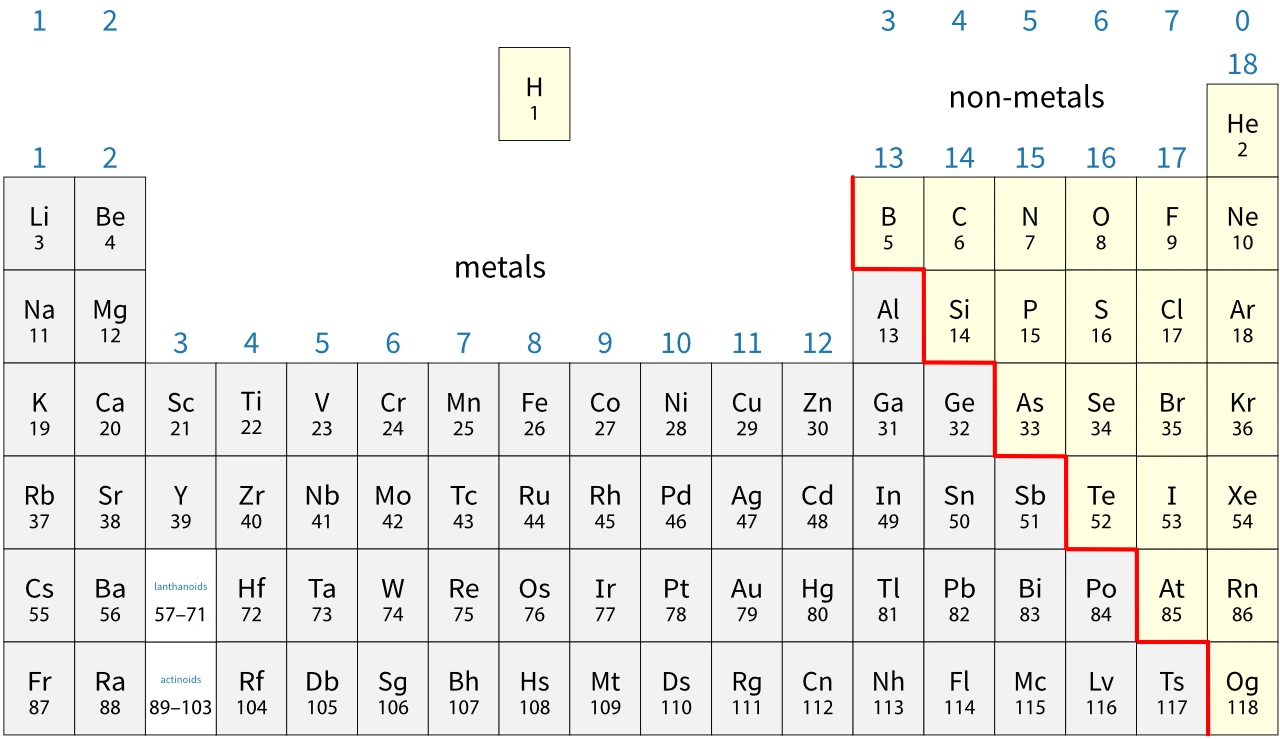
Where are the metals and non- metals on the periodic table
All elements in the separated bottom section are also metals .
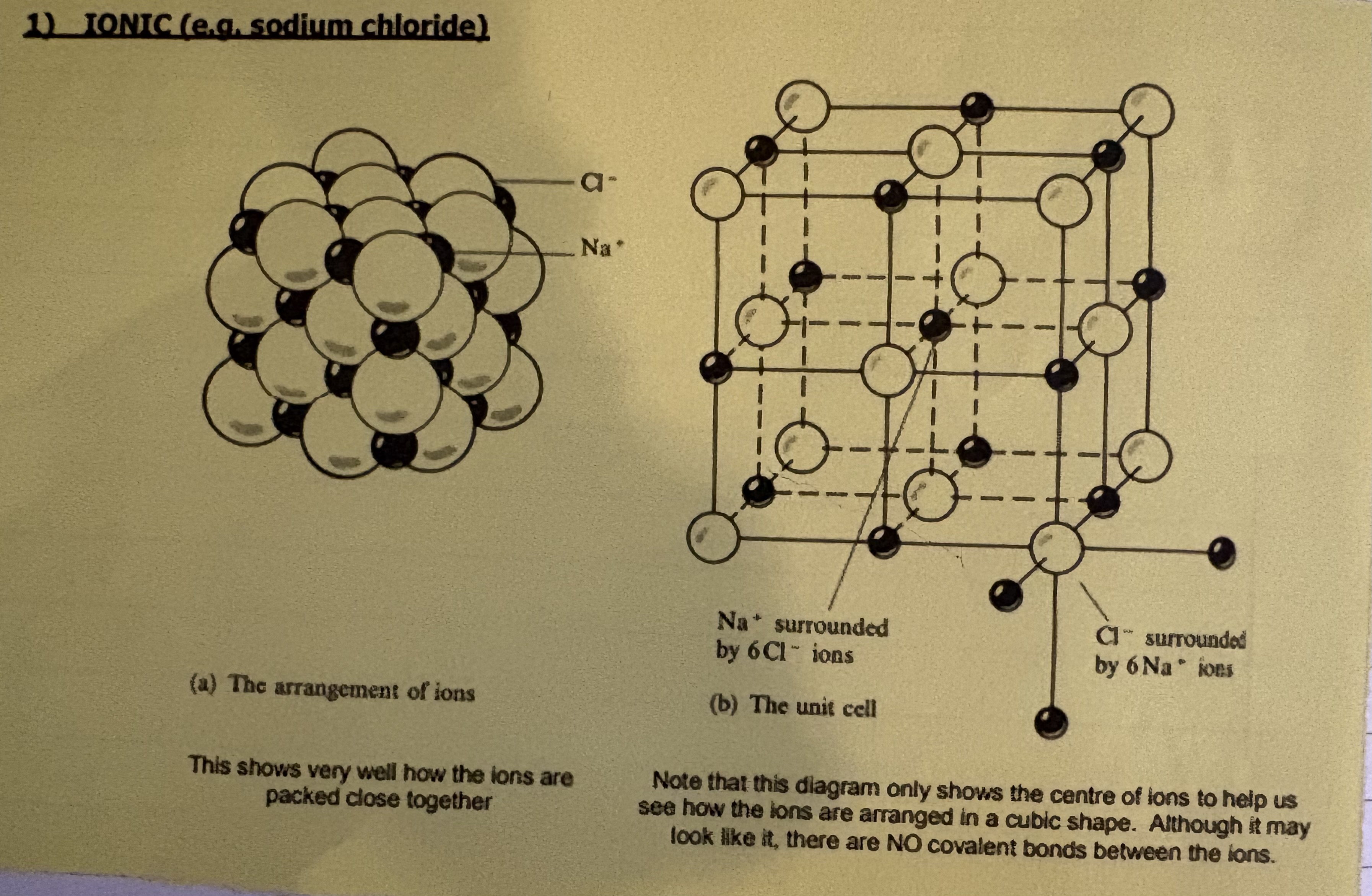
What are the two ways to model ionic compounds?
What is the definition of ionic bonding ?
The electrostatic attraction between two oppositely charged positive and negative ions .
What does electrostatic attraction mean ?
The forces of attraction between positive and negative ions
What are valance electrons/ the valance shell ?
The outer electrons or shell that are involved in bonding.
What happens to the electrons in ionic bonding ?
They are transferred
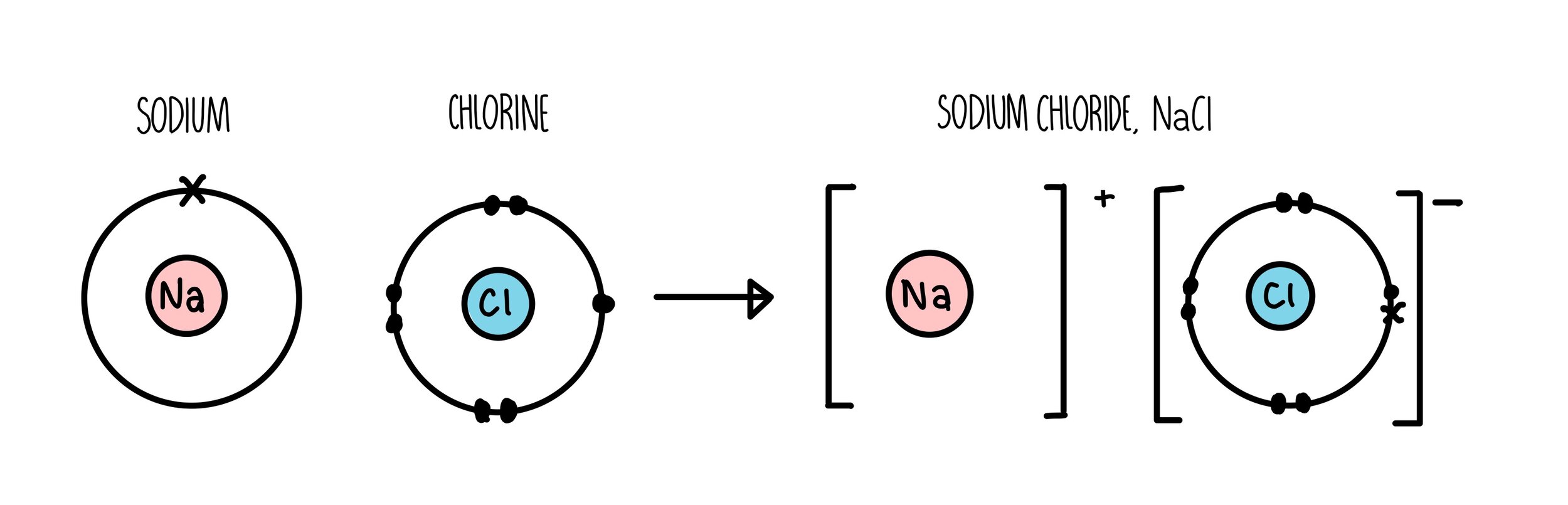
How do you draw ionic bonds ?
The positively charged element (metal) is left with a naked shell
The negatively charged element (non-metal) has it’s own electrons in the valance shell represented with crosses , and the electrons transferred to it with dots to get a full outer shell
Put the elements in square brackets with the charge outside
Ensure the charges cancel out to 0/ the outer shell is full on one and empty on another by having multiple of elements if needed . To do this put a large number before the square brackets if that element
What does isoelectronic mean ?
Same electronic structure / same number of electrons