Atomic structure
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
What is Heisenberg’s uncertainty principle?
It is impossible to simultaneously know the exact position and speed of an electron
Scrondinger’s wave equation
An equation that can calculate the energy level of an electron
How does Schrodinger describe electrons?
As a standing wave
Pauli’s exclusion principle
Each orbital can have 2 electrons with the same n,l,m values but must have opposite spin numbers (1/2 OR -1/2)
VESPR theory
Method to predict the 3D structure around the atom (the structure of the compound)
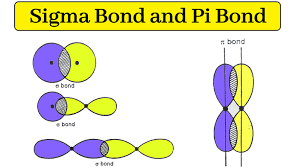
Sigma bond
When the tips of 2 orbitals directly overlap
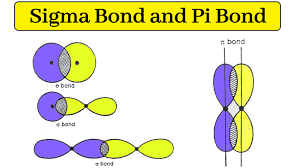
Pi bonds
When two orbitals overlap side by side
True or false: the weaker the intermolecular forces the higher the melting and boiling points
False, the stronger the intermolecular forces the higher the melting and boiling points
True or false: london dispersion force is a weak attractive force between two non polar molecules
True
True or false: hydrogen bonds are the strongest bonds
True
A EN<0.5 = _________
non polar
a EN > 0.5 = ______
Polar
Aufbau"‘s principle
Electrons fill the lowest energy levels first.
Hans rule
Electrons fill each orbital singly before pairing up.