MCAT Bonding and Chemical Interactions
1/85
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
86 Terms
molecules
Groups of two or more atoms held together by chemical bonds
chemical bonds
the attractive forces that hold atoms together via the interaction of the valence electrons
octet rule
States that atoms lose, gain or share electrons in order to acquire a full set of eight valence electrons similar to noble gases; many exceptions to the rule
exceptions to the octet rule
Hydrogen (2), Boron (3-6), Lithium (2) and beryllium (4) Aluminum(sometimes 6, but 3), Phosphorus(10), Sulfur(up to 12). All elements in period 3 and greater can expand the valence shell to include more than eight electrons by incorporating d-orbitals.
incomplete octet
These elements are stable with fewer than eight electrons in their valence shell and include hydrogen (2), helium (2), lithium (2), beryllium (4), and boron (6).
expanded octet
any element in period 3 and greater can hold more than eight electrons, including phosphorous (10), sulfur (12), chlorine (14), and many others
odd number of electrons
any molecule with odd number of valence electrons cannot distribute those electrons to give eight to each atom; for example nitric oxide NO has eleven valence electrons
common elements that almost always abide by the octet rule
carbon, nitrogen, oxygen, fluorine, sodium, and magnesium
Nonmetals __________ electrons to form ____________
gain (anion); complete octets
Metals ________ electrons to form ____________
lose (cation); complete octets
anion
A negatively charged ion
cation
A positively charged ion
2 types of chemical bonds
ionic and covalent
ionic bonding
one or more electrons from an atom with low ionization energy, typically a metal, are transferred to an atom with a high electron affinity, typically a nonmetal. Held together by resulting electrostatic attractions between opposite charges.
EX: NaCl
covalent bonding
an electron pair is shared between two atoms, typically nonmetals, that have relatively similar values of electronegativity. The degree to which the pair of electrons is shared equally or unequally between the two atoms determines the degree of polarity
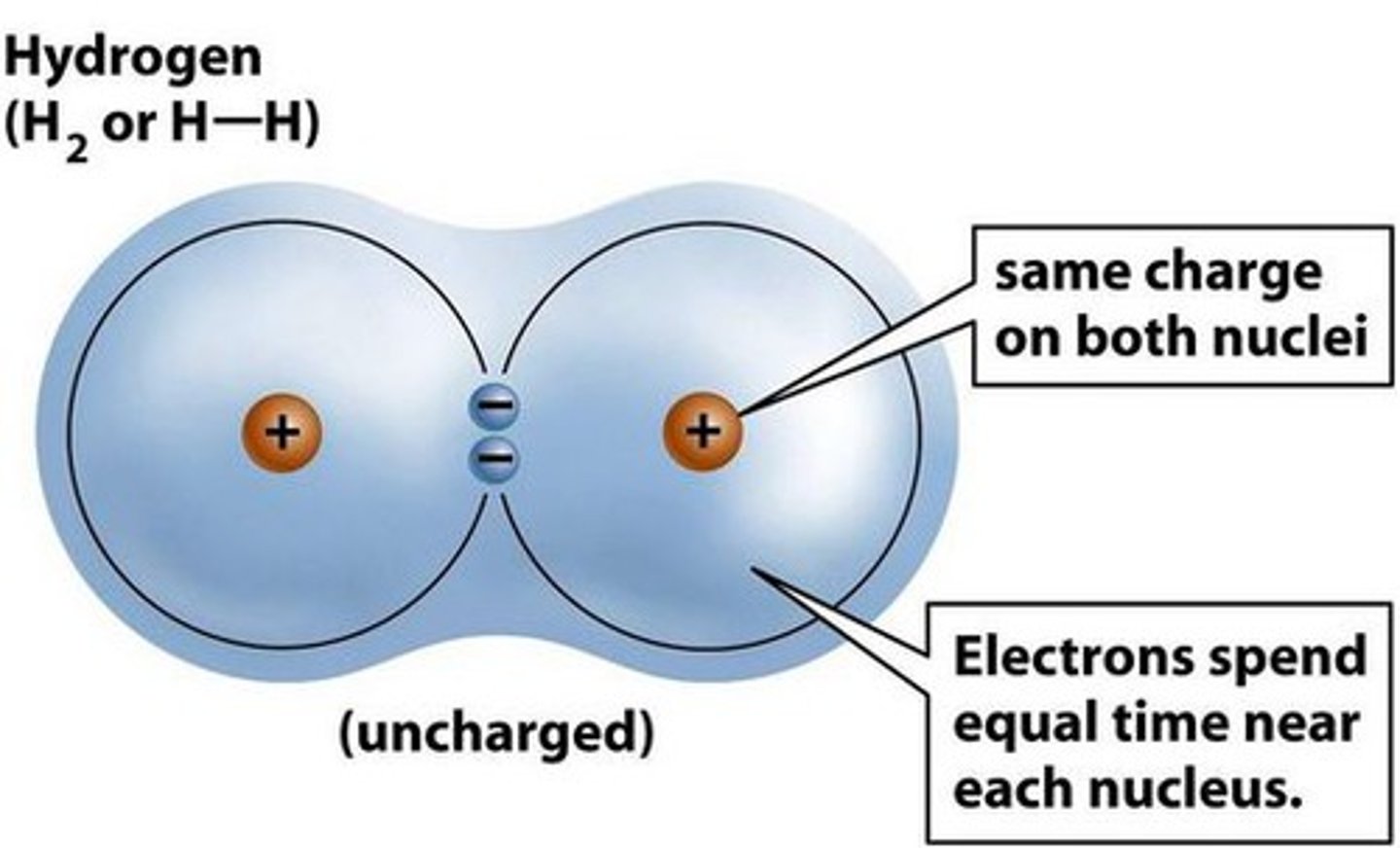
nonpolar covalent bond
a covalent bond in which the electrons are shared equally by the two atoms
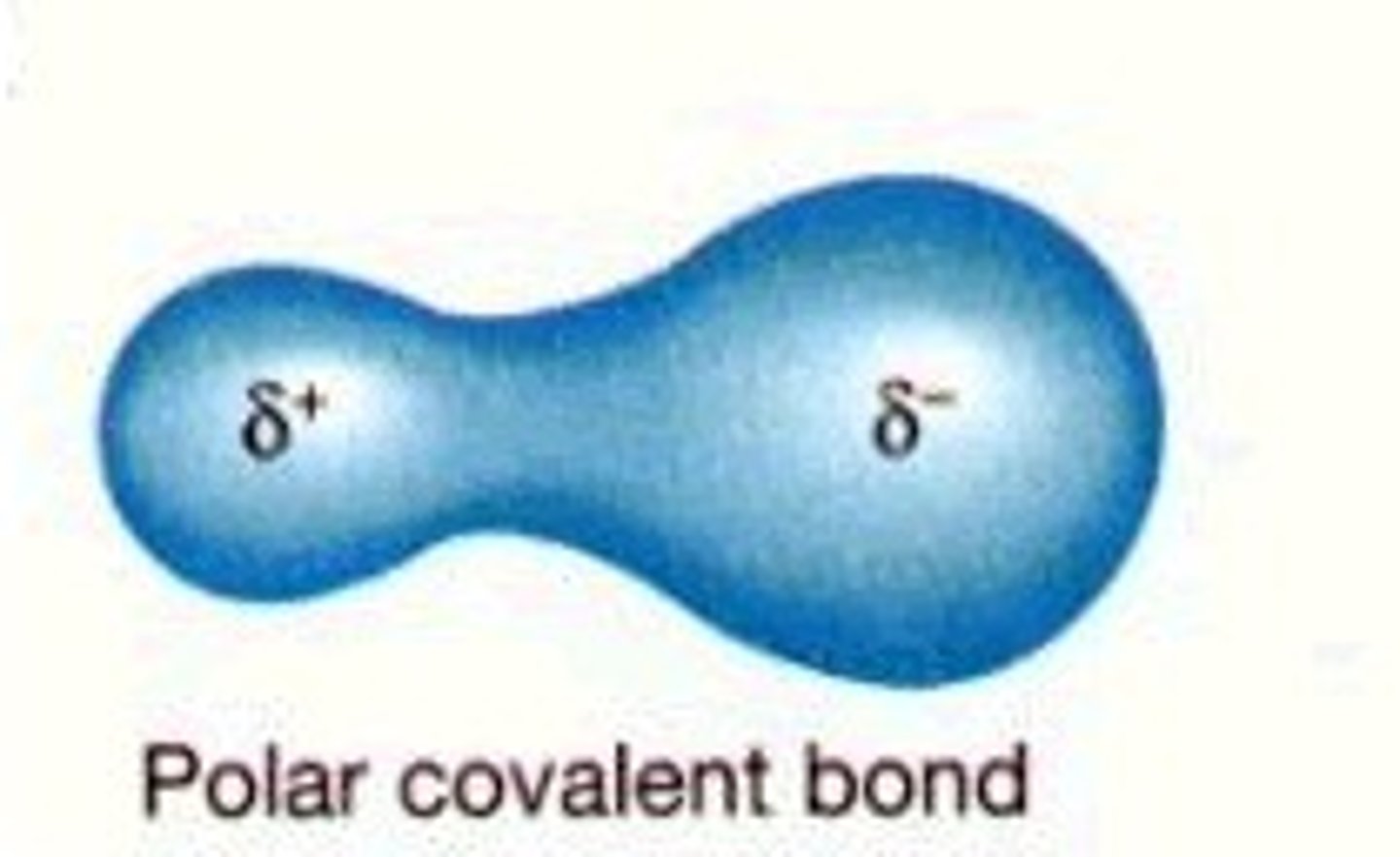
polar covalent bond
A covalent bond in which electrons are not shared equally
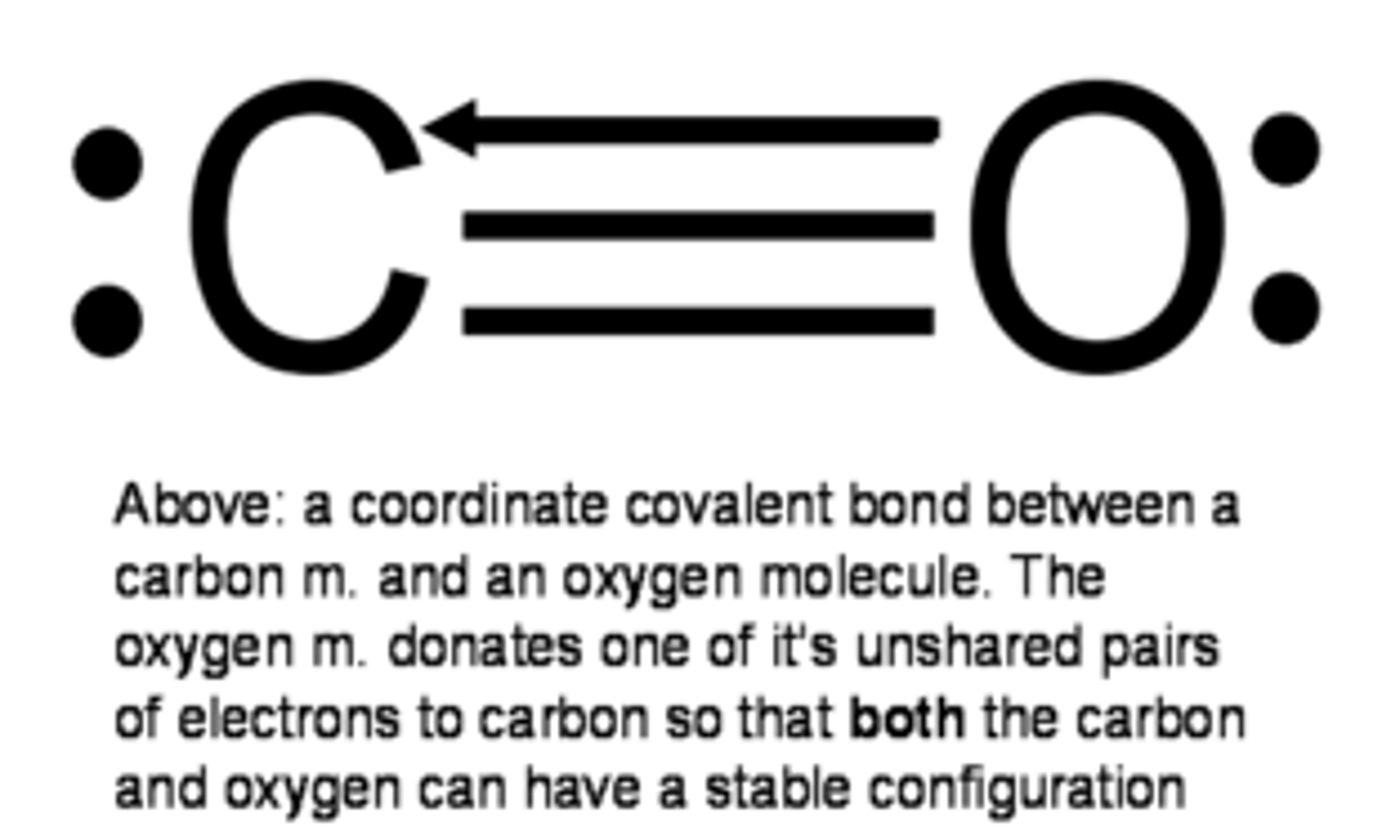
coordinate covalent bond
a covalent bond in which one atom contributes both bonding electrons
Polarity is determined by
difference in electronegativity
meTals lose electrons to become
caTions = posiTive ions
Nonmetals gain electrons to become
aNions = Negative ions
for electrons transfer to occur
the difference in electronegativity must be greater than 1.7 on the Pauling scale
ionic bonds are generally formed between
metals and nonmetals; metals have low ionizations energies (give up electrons readily) and nonmetals have high electron affinities (gain electrons)
The nonmetals "wins" the tug of war and becomes an anion by gaining electrons.
characteristics of ionic bonds
very high melting point
very high boiling point
many dissolve readily in water and other polar solvents
good conducts of electricity in molten or aqueous state
form crystalline lattice (attractive forces maximized and the repulsive forces minimized)
crystalline lattice
Regular geometric shape found in a solid in which the component particles are arranges in an orderly, three-dimensional, repeating pattern.
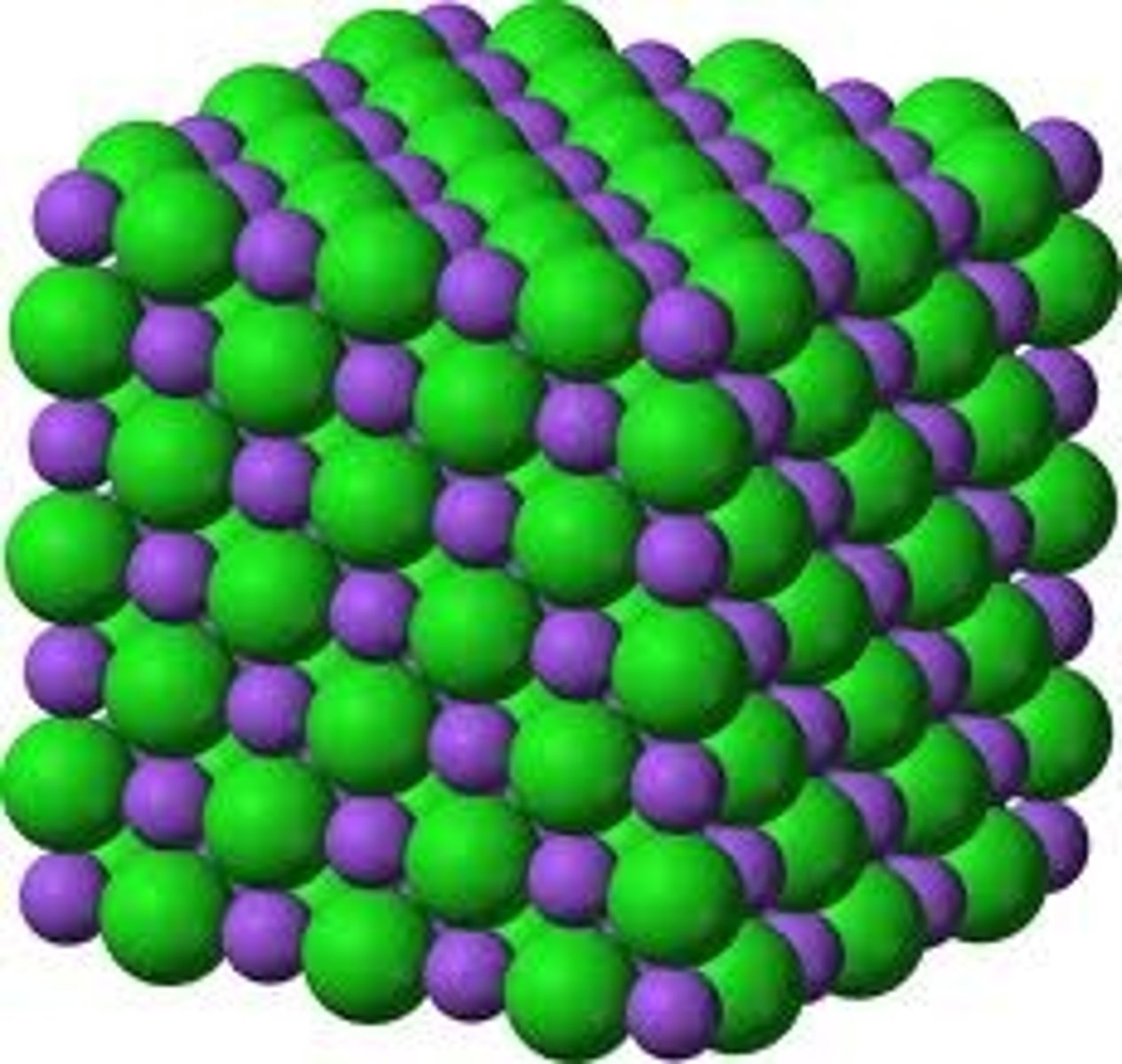
characteristics of covalent bonds
weaker bonds with lower melting and boiling points; poor conductors of electricity in liquid due to being insoluble.
bond order
the number of shared electron pairs between two atoms; electrons are joined by single (bond order = 1), double (bond order = 2), or triple (bond order =3) covalent bonds.
bond length
the average distance between the nuclei of two bonded atoms; decreases as the number of shared electron pairs increases.
Triple < double < single
bond energy
the energy required to break a chemical bond and form neutral isolated atoms; the greater the number of pairs of electrons shared between the atomic nuclei, the more energy is required to break the bonds holding the atoms together.
single < double < triple
greater bond energy = stronger bond
polarity
-occurs when 2 atoms have a difference in electronegativity
-atom with higher EN gets more e-
-A polar bond creates a dipole --> + end at least EN atom and - end at most EN atom
-EN of 0.5-1.7 are polar
- EN of less than 0.5 are nonpolar
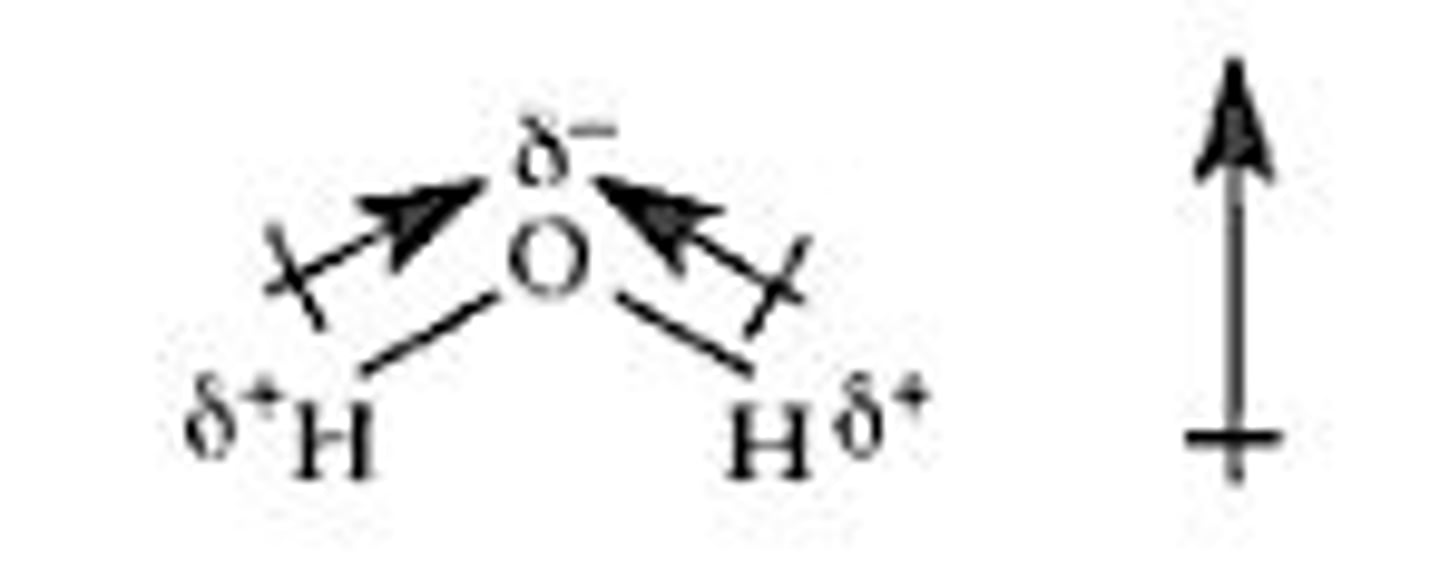
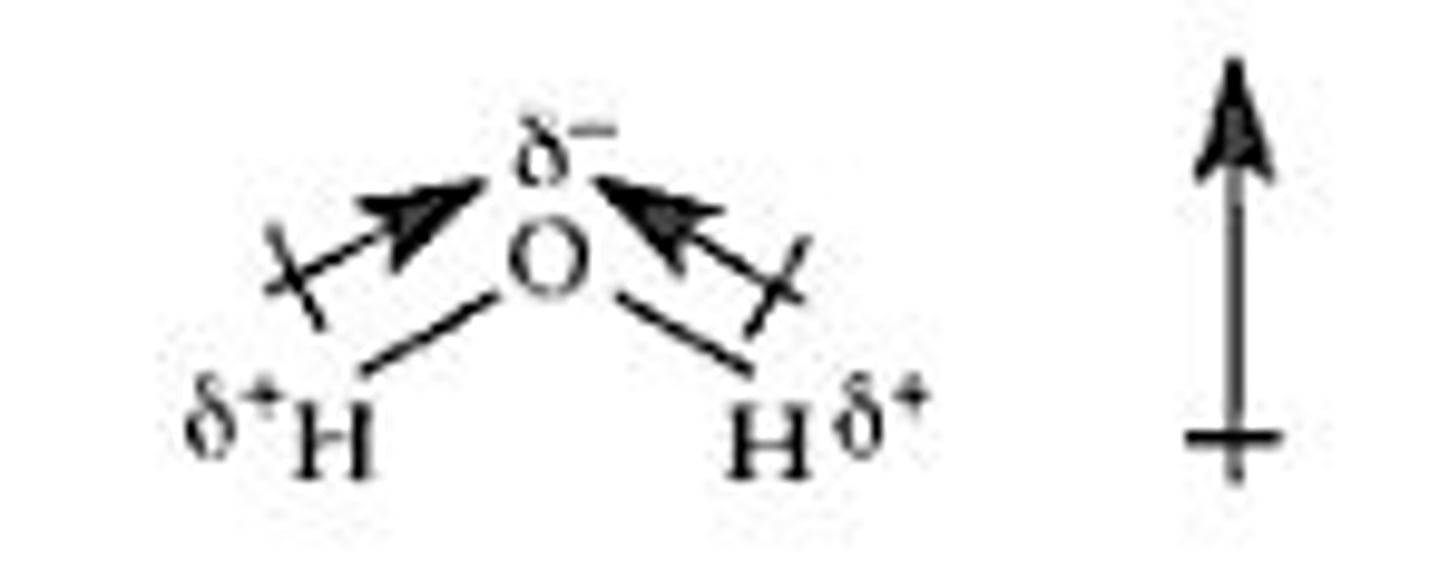
dipole
created by equal but opposite charges that are separated by a short distance; positive end on the less electronegative atom and negative end on the more electronegative atom.
naturally occurring diatomic elements
H2, N2, O2, F2, Cl2, Br2, I2
form the number 7 on the periodic table (except for H), there are 7 of them, and most of them are in group VIIA
electronegativity range for nonpolar bonds
0 to 0.5
electronegativity range for polar bonds
0.5 to 1.7
electronegativity range for ionic bonds
1.7 and greater
partial negative charge
A small electrically negative charge on the end of a polar molecule.
partial positive charge
A small electrically positive charge on the end of a polar molecule.
dipole moment
a property of a molecule whose charge distribution can be represented by a center of positive charge and a center of negative charge
dipole moment equation
p=qd
p is dipole moment
q is the magnitude of the charge
d is the displacement vector
Lewis acid
an atom, ion, or molecule that accepts an electron pair to form a covalent bond
Lewis base
an atom, ion, or molecule that donates an electron pair to form a covalent bond
bonding electrons
pairs of valence electrons that are shared between atoms in a covalent bond
nonbonding electrons
(lone pairs) the valence electrons in a molecule that are not shared
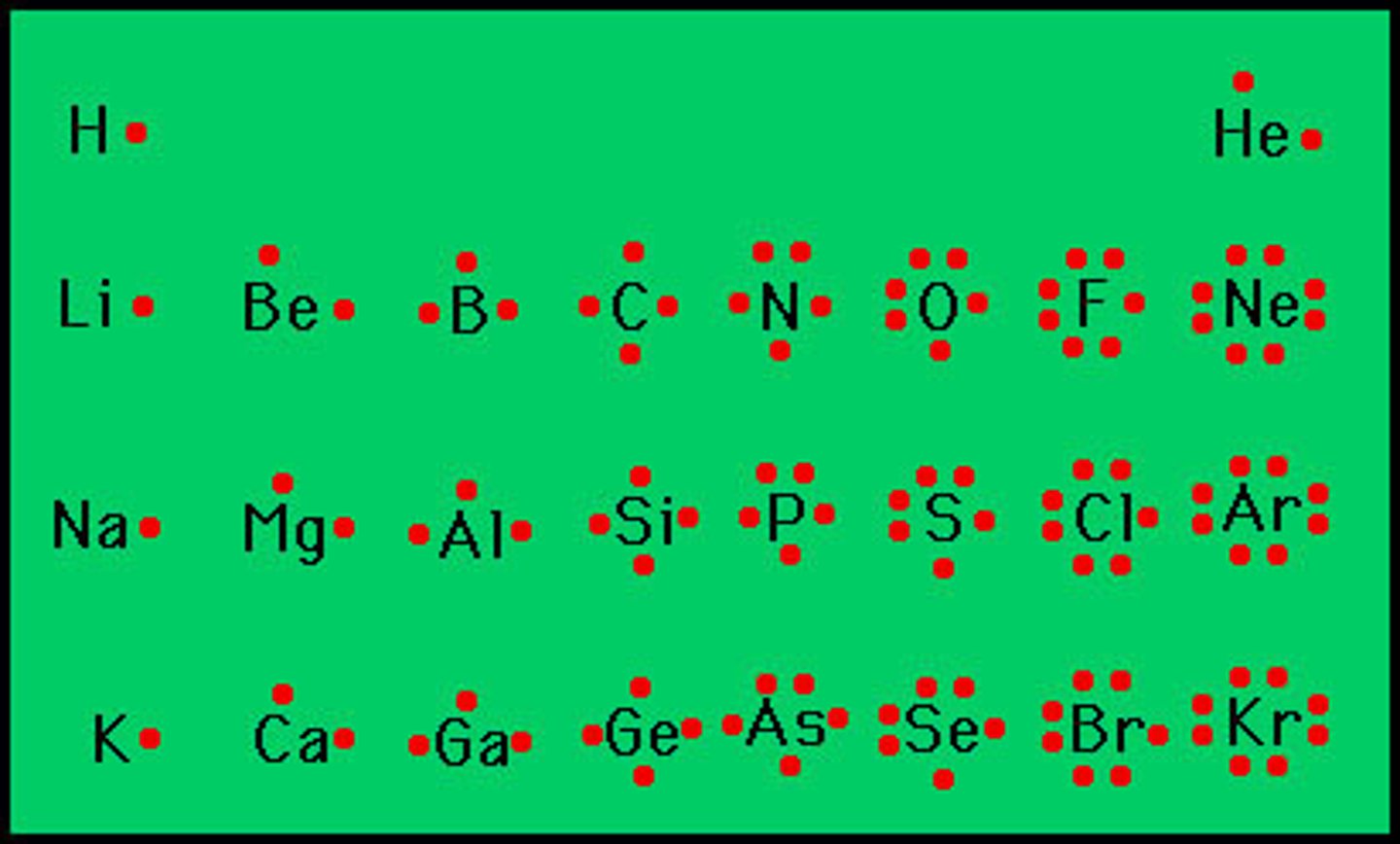
Lewis structure
A model that uses electron-dot structures to show how electrons are arranged in molecules. Pairs of dots or lines represent bonding pairs; each dot representing one of the s or p valence electrons of the atom (some atoms, those in period 3 or higher, can expand their octet by utilizing d-orbitals)
formal charge
The number of valence electrons in an isolated atom minus the number of electrons assigned to the atom in the Lewis structure
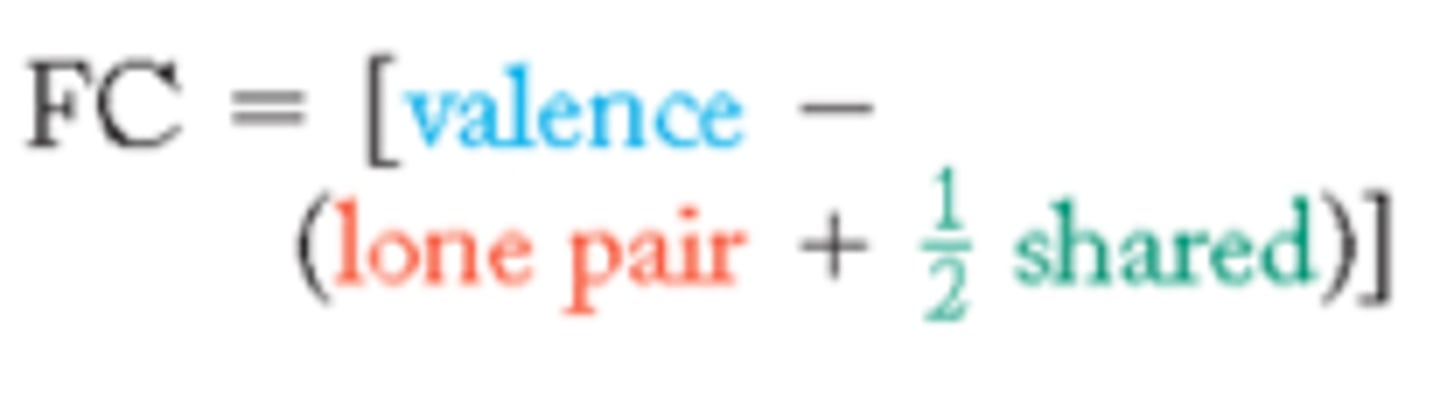
the arrangement of the Lewis structure that _______ the number and magnitude of formal charges is usually the most stable arrangement of the compound
minimizes
steps for drawing a Lewis structure with HCN as an example
1. Draw out the backbone of the compound, with the least electronegative atom as the central atom. Hydrogen and the halogens occupy terminal positions.
H-C-N
2. Count all the valence electrons of the atom. the number of valence electrons of the molecule is the sum of the valence electrons of all atoms present.
H = 1
C = 4
N = 5
HCN = 10
3. Draw single bond between the central atom and the atoms surround it. Each single bond corresponds to a pair of electrons.
H:C:N
4. Complete the octet of all of the atoms bonded to the central atom.
H:C:N:::
5. Place any extra electrons on the central atom. If there are none and the central atom has less than an octet, form double or triple bonds.
H-C(triple bond)N:
formal charge equation
FC = V - N_nonbonding - 1/2 N_bonding
V = is the normal number of electrons in the atom's valence shell
N_nonbonding = number of nonbonding electrons
N_bonding = the number of bonding electrons (double the number of bonds)
the charge of an ion or compound =
the sum of the formal charge of the individual atoms comprising the ion or compound
calculate the formal charge of N in [NH_4]+
FC = 5 - 0 - (1/2)(8) = +1
resonance
the bonding in molecules or ions that cannot be correctly represented by a single Lewis structure; it allows for greater stability, delocalizing electrons and charges over what is known as a pi system.
resonance structures
Lewis structures that have the same arrangement of atoms in a molecule but differ in the distribution of electrons among the atoms.

resonance hybrid
-structure of a compound whose electronic distribution is made of all of the possible resonance structures
-the more stable the structure, the more it contributes to the hybrid
stability of resonance structures
- a structure with small or no formal charge is preferred.
- a structure with less separation between opposite charges is preferred.
- a structure in which negative formal charges are placed on more electronegative atoms is more stable.
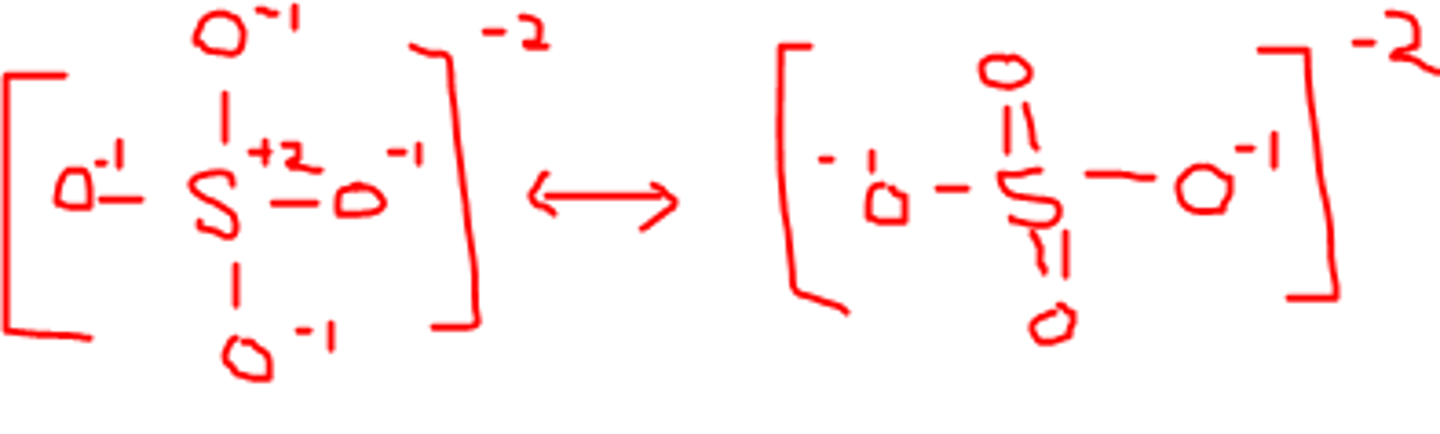
resonance structure of sulfate ion
Valence Shell Electron Pair Repulsion (VSEPR) Theory
reflects the actual geometric arrangement of atoms in an element.
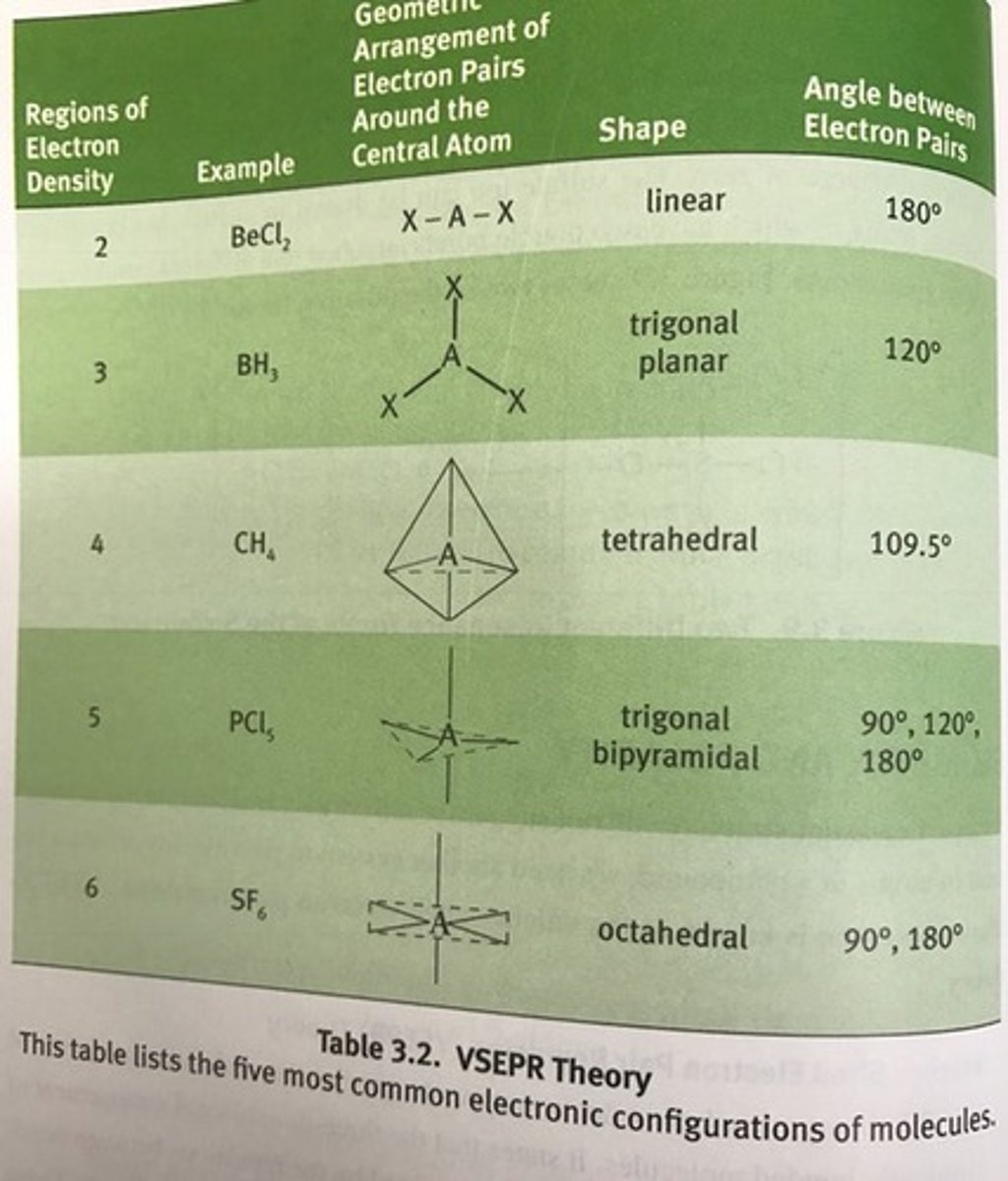
steps to predict the geometrical structure of a molecule using the VSEPR theory
1. Draw the Lewis dot structures of the molecule.
2. Count the total number of bonding and nonbonding electron pairs in the valence shell of the central atom.
3. Arrange the electron pairs around the central atom so they are far apart as possible.
atoms that are involved in molecules that I need to be prepared to draw or identify structural formulas for:
H, C, N, O, F, S, P, Si, Cl
Focus on being able to create a Lewis diagram for any element and predicting its three-dimensional shape from VSEPR theory.
electronic geometry vs molecular geometry
electronic geometry describes the spatial arrangement of all pairs of electrons around the central atom, both bonding and lone pairs
-molecular geometry describes the spatial arrangement of only the bonding pairs of electrons
ex. NH3
electronic G= tetrahedral
molecular G= trigonal pyramidal
*presence of lone pair on the central atom makes for a different shape between electronic and molecular geometries.
electronic geometry
-spatial arrangement of all pairs of e- around central atom (both bonding e- and lone pairs of e-)
molecular geometry
the arrangement of bonded atoms
coordination number
the number of atoms that surround and are bonded to a central atom and is the relevant factor when determining the molecular geometry.
linear shape
180 degrees
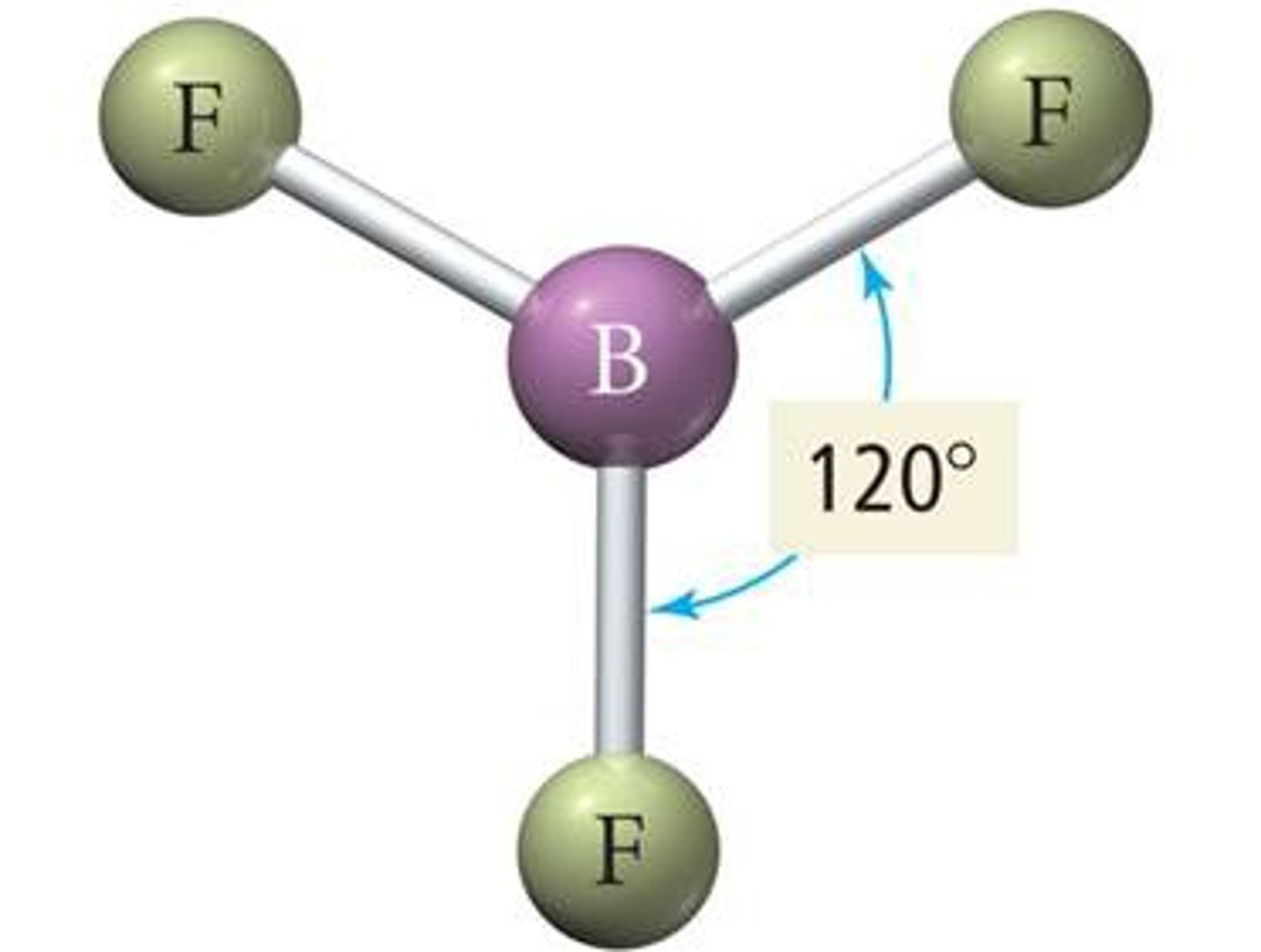
trigonal planar
120 degrees
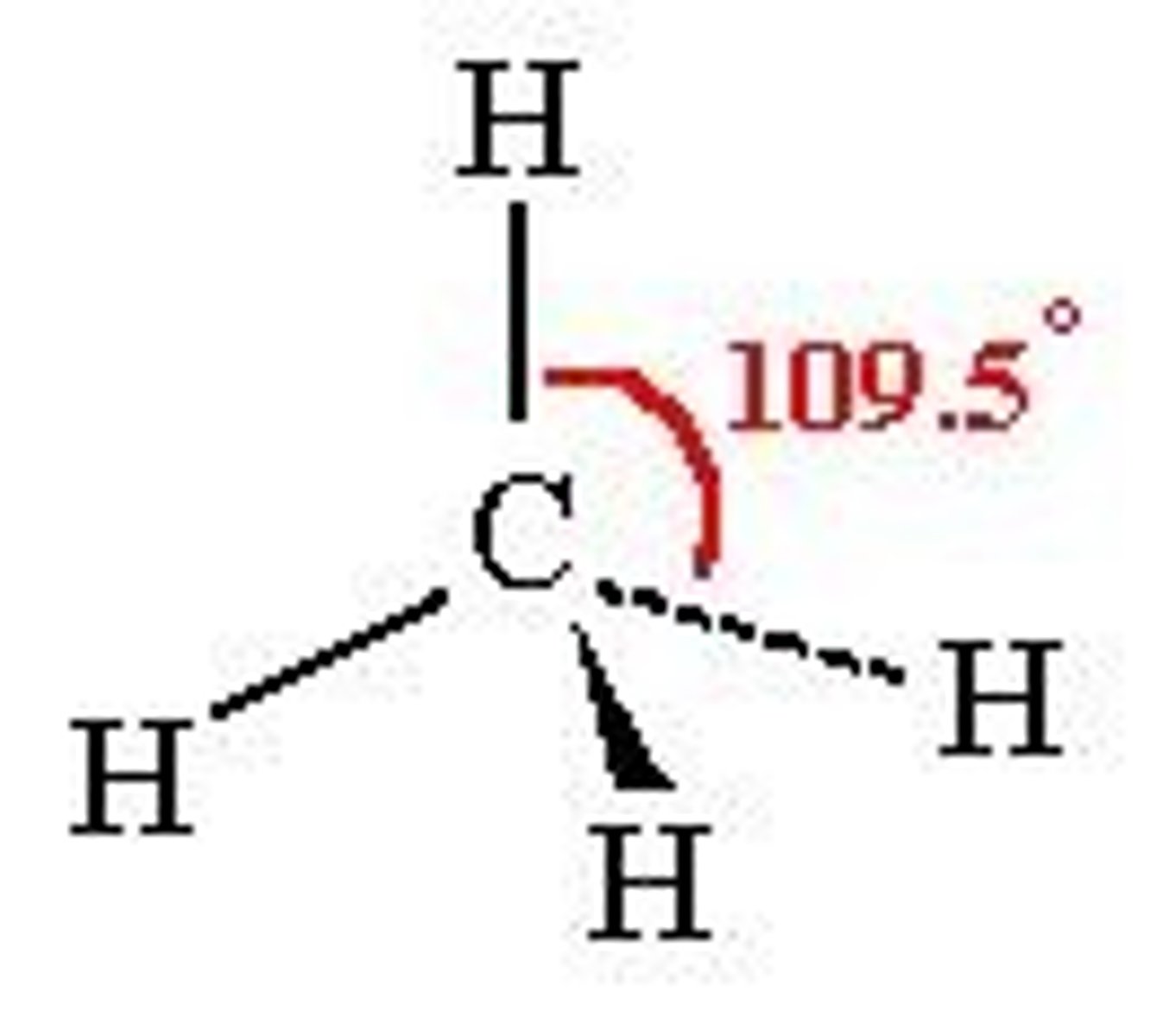
Tetrahedral
109.5
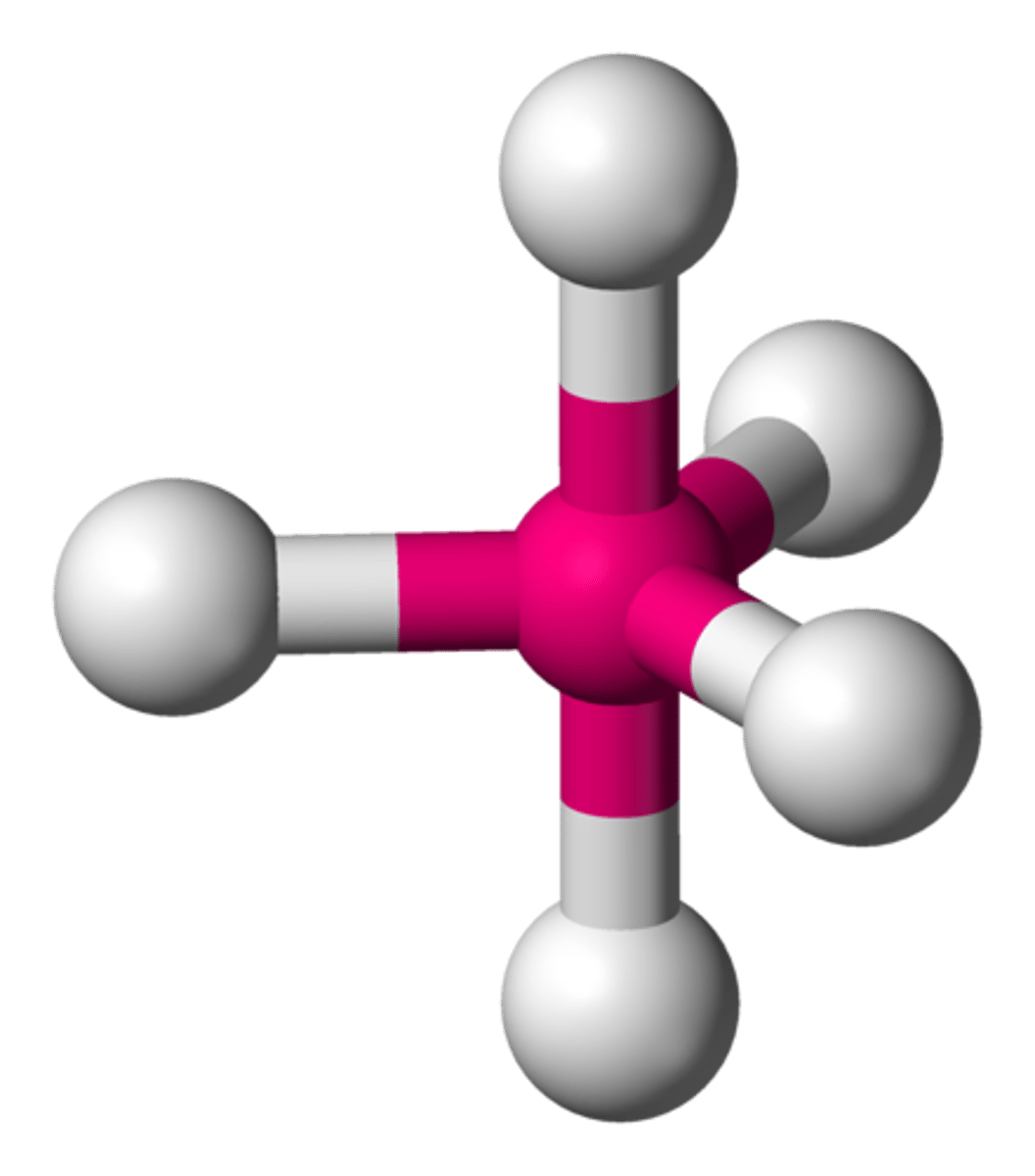
trigonal bipyramidal
90, 120, 180
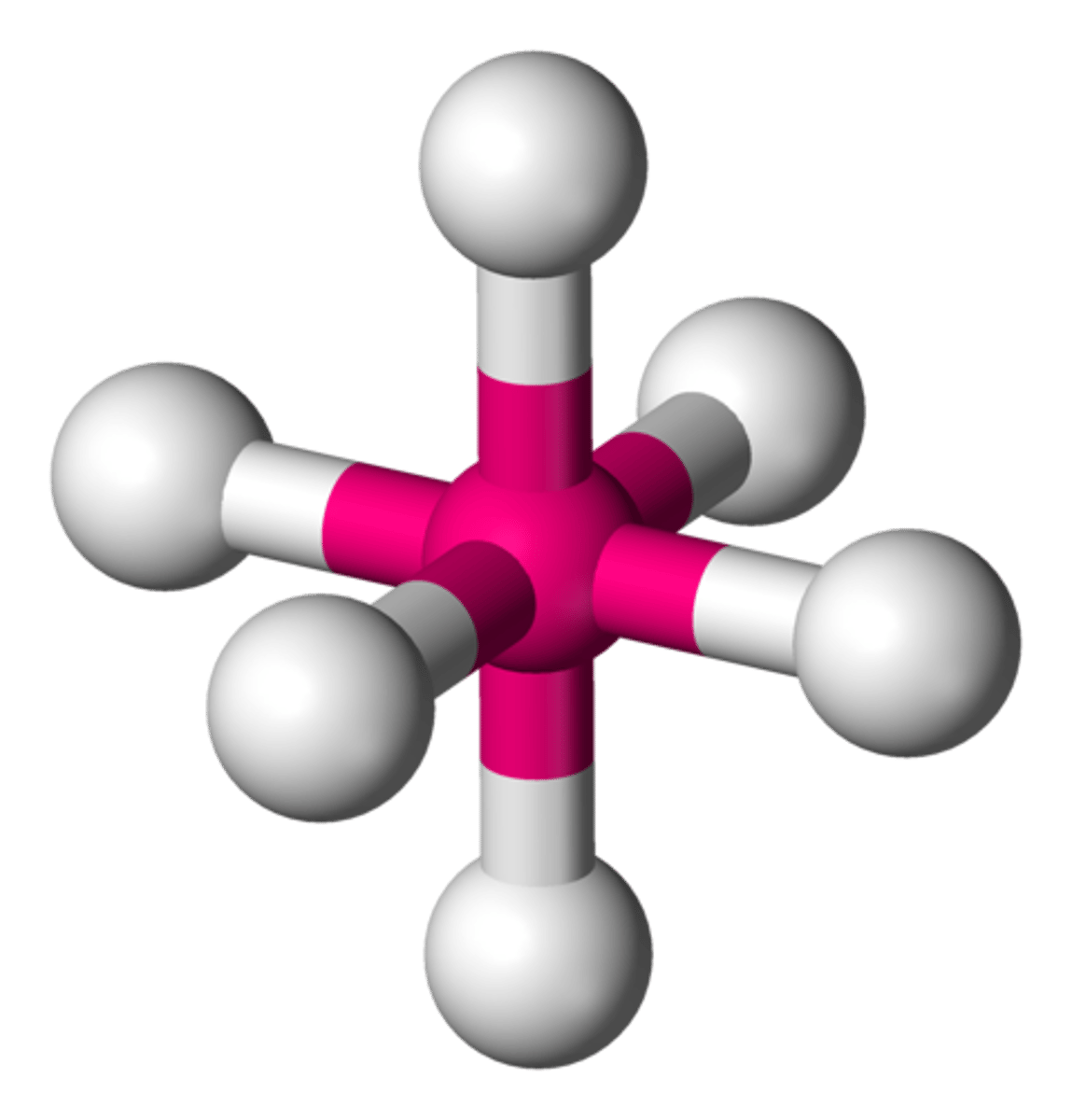
octahedral
90, 180
ideal bond angle
-determined by electronic geometry
-lone pairs of e- are closer to the nucleus so they exert more repulsion
-presence of lone pairs of e- makes ideal angle smaller
net dipole moment
the sum of all dipole moments present in an ion or molecule.
Polar bonds with dipole moments can cancel out to have no net dipole moment and are therefore nonpolar.
No polar dipole moments are always nonpolar but polar dipole moments are not always polar.
compound vs molecule
A molecule is formed when two or more atoms join together chemically. A compound is a molecule that contains at least two different elements. All compounds are molecules but not all molecules are compounds.
vector summation
The process of combining two or more vectors into a single vector
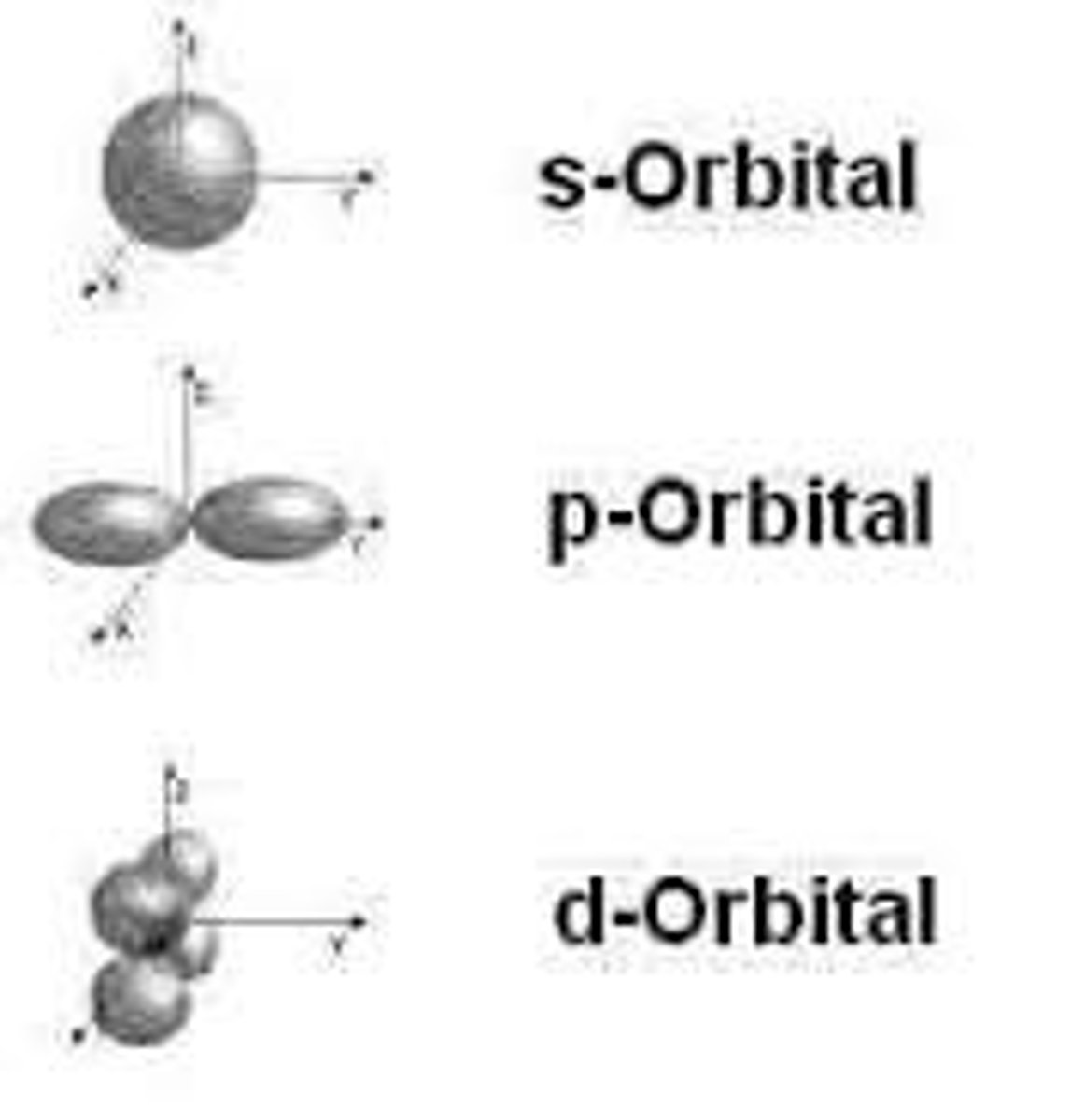
orbital shapes
focus on s and p
molecular orbital
When two atoms combine, their atomic orbitals overlap to produce orbitals that apply to the entire molecule and describes the probability of finding the bonding electrons in a given space.
bonding orbital
a molecular orbital that can be occupied by 2 electrons of a covalent bond if the signs of the two atomic orbitals are the same.
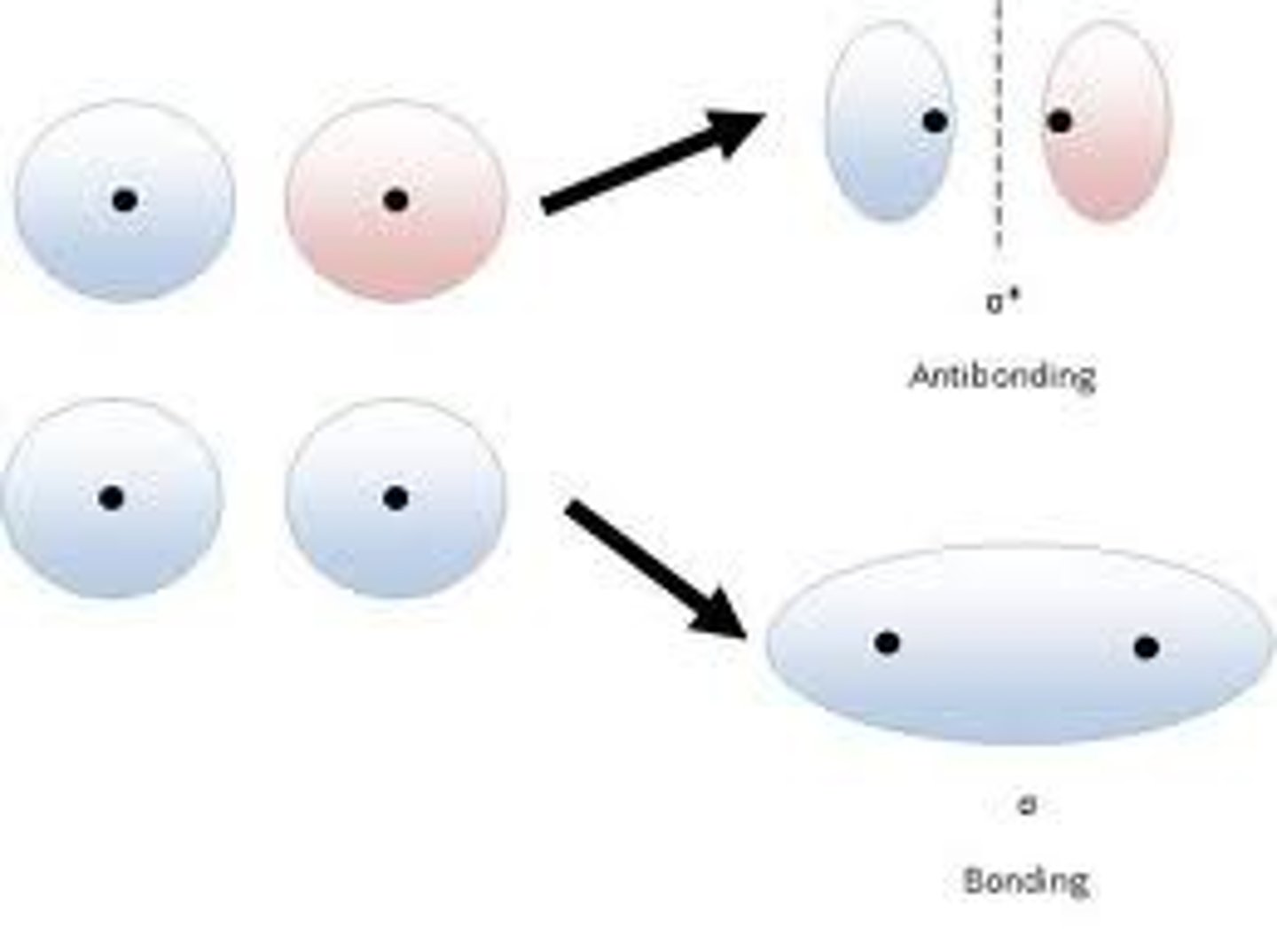
antibonding orbitals
created by head-to-head or tail-to-tail overlap of atomic orbitals that have opposite signs and are energetically unfavorable
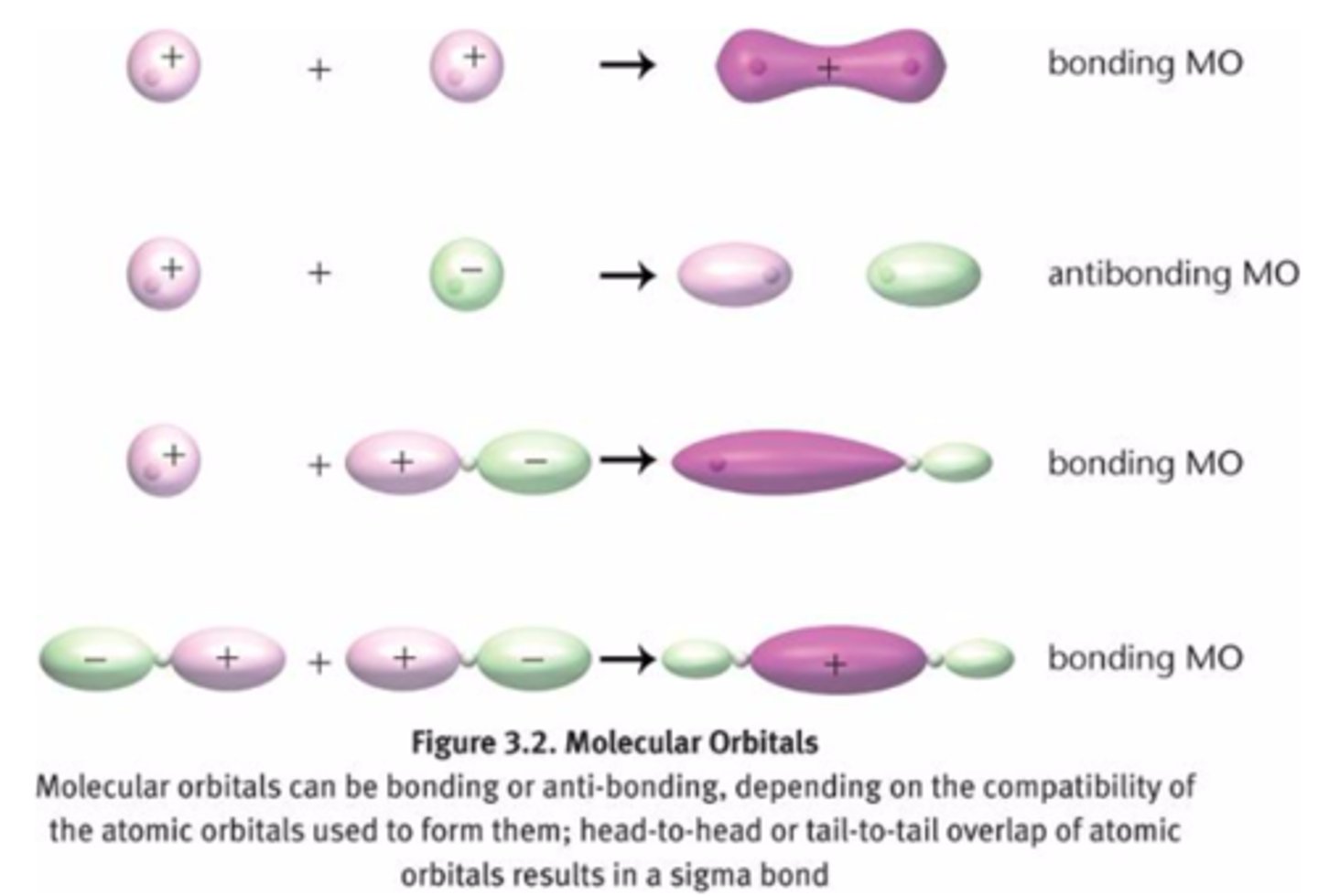
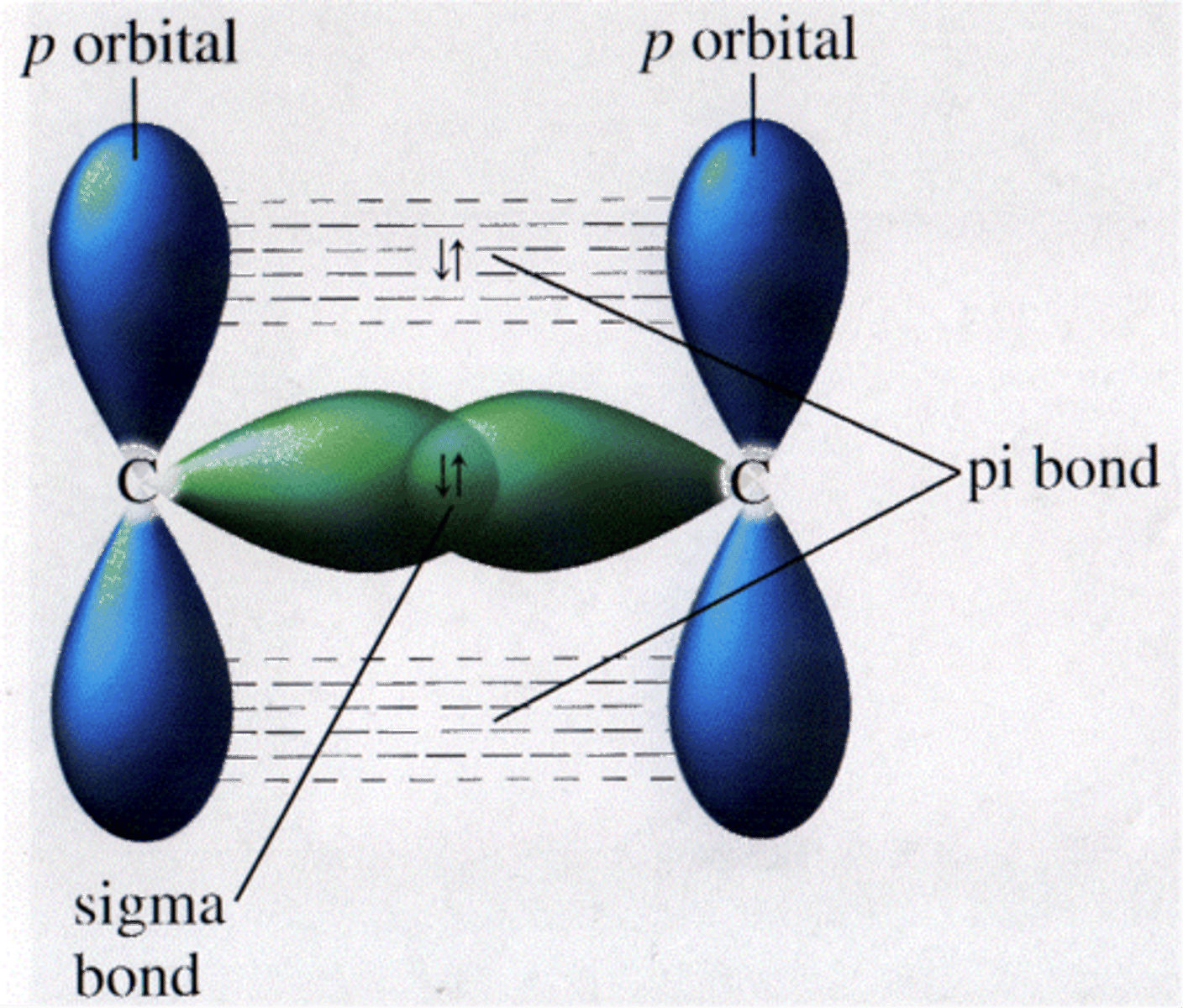
sigma bond
a single covalent bond that is formed when an electron pair is shared by the direct overlap of bonding orbitals head to head. Allows for free rotation about their axes because the electron density of the bonding orbitals is a single linear accumulation between the atomic nuclei.
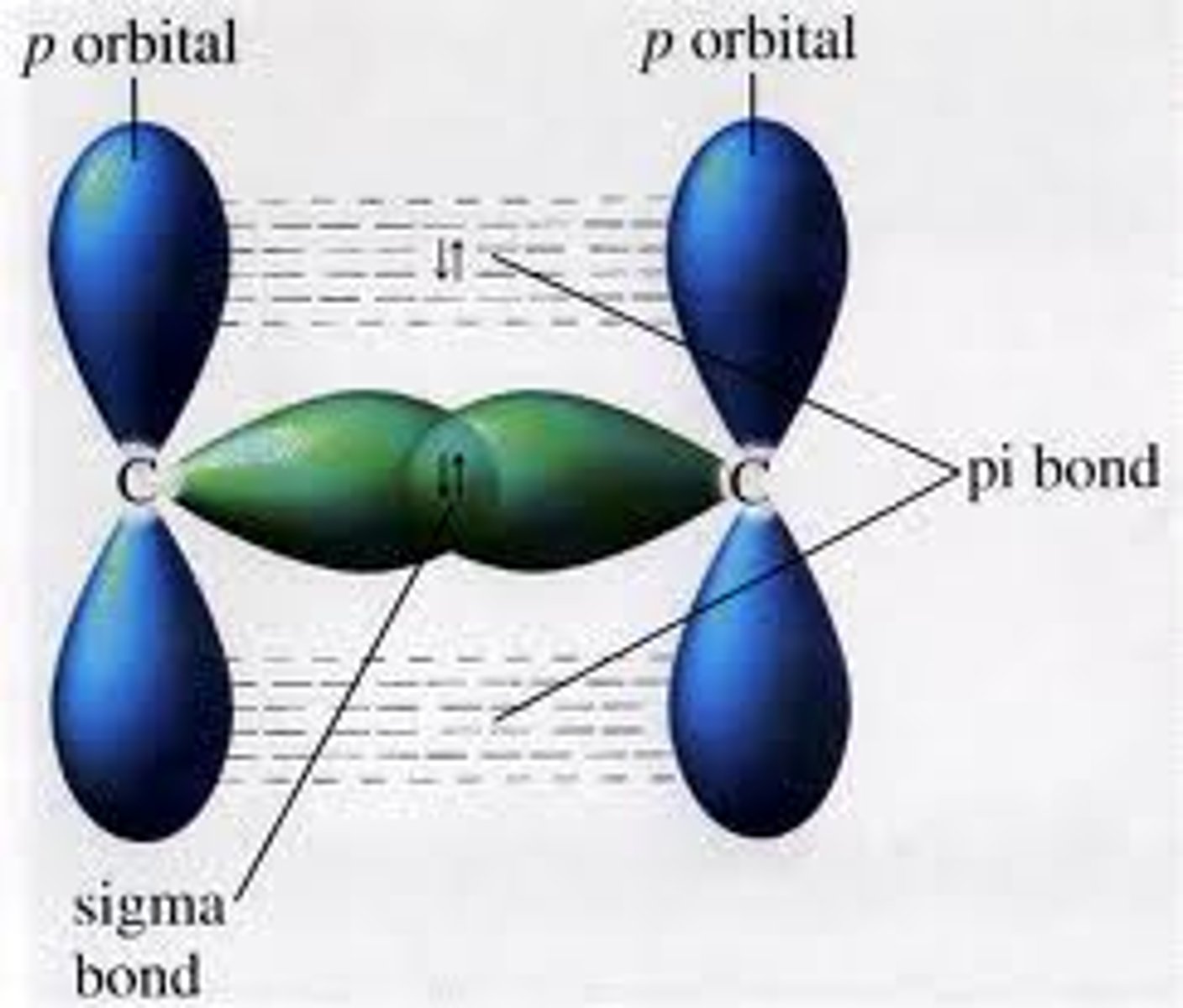
pi bond
when the orbitals overlap in such a way that there are tow parallel electron cloud densities. Does not allow for free rotation.
intermolecular forces
attractive forces between molecules
dispersion forces
weak forces that result from temporary shifts in the density of electrons in electron clouds; weaker than covalent bonds and easily overcome
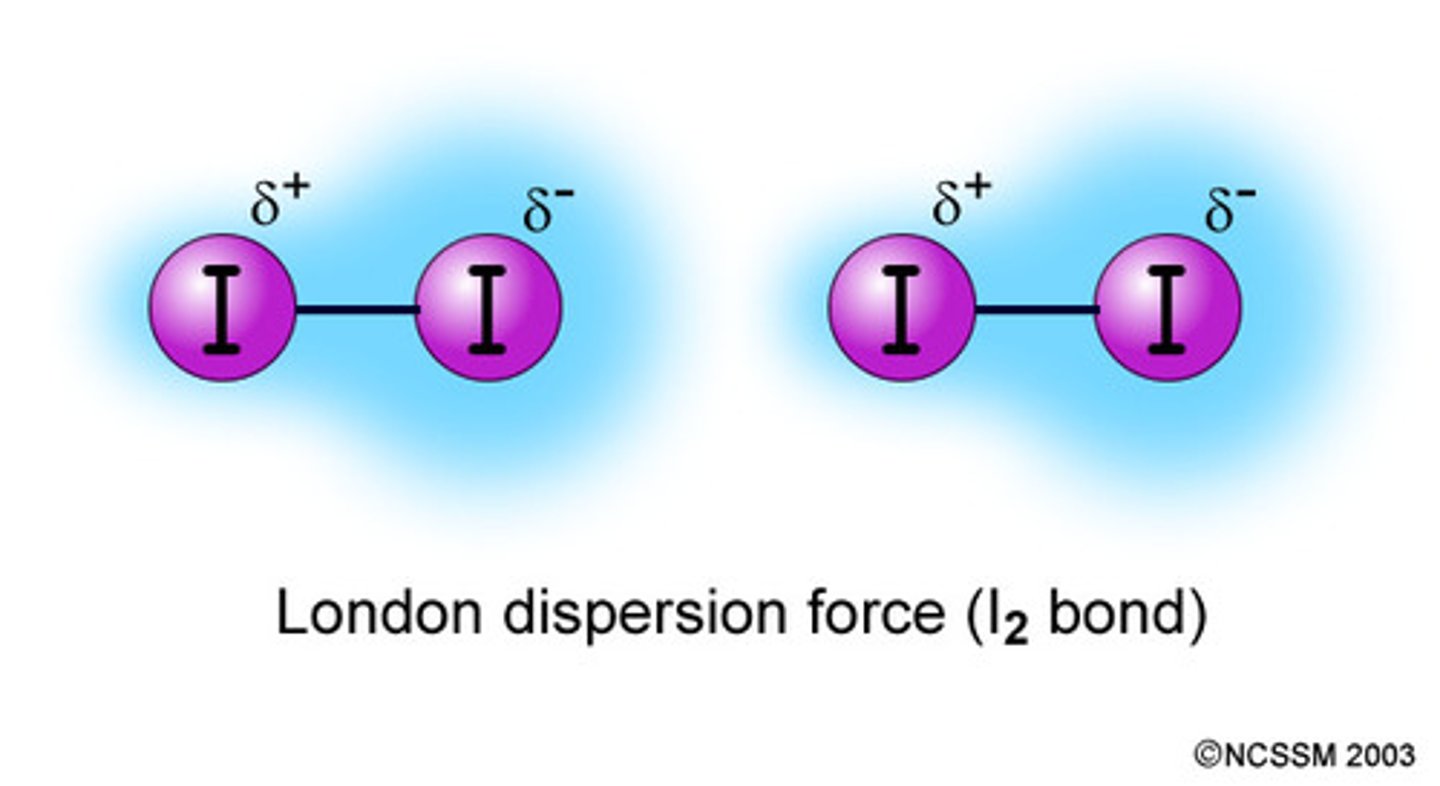
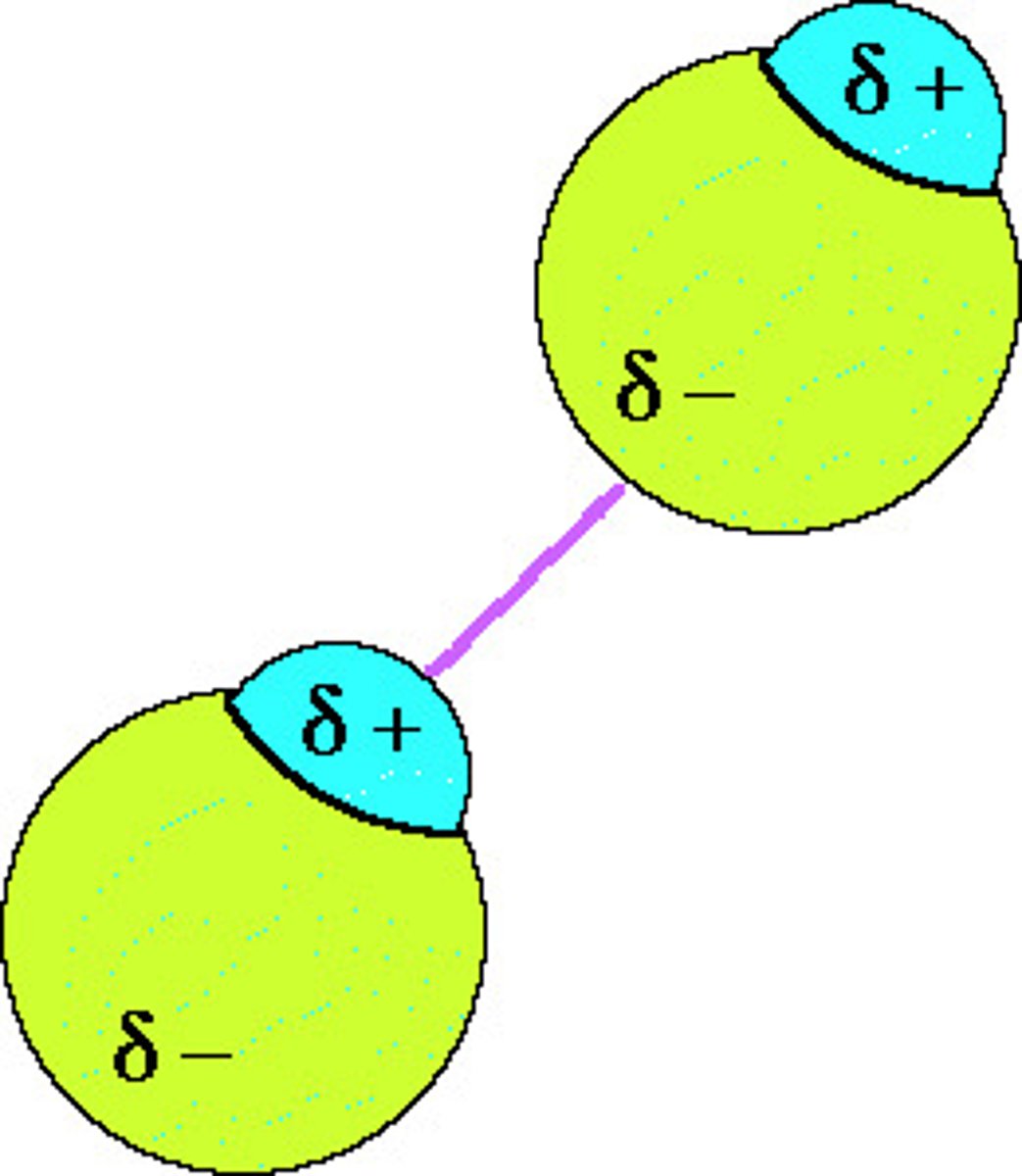
dipole-dipole forces
attractions between oppositely charged regions of polar molecules
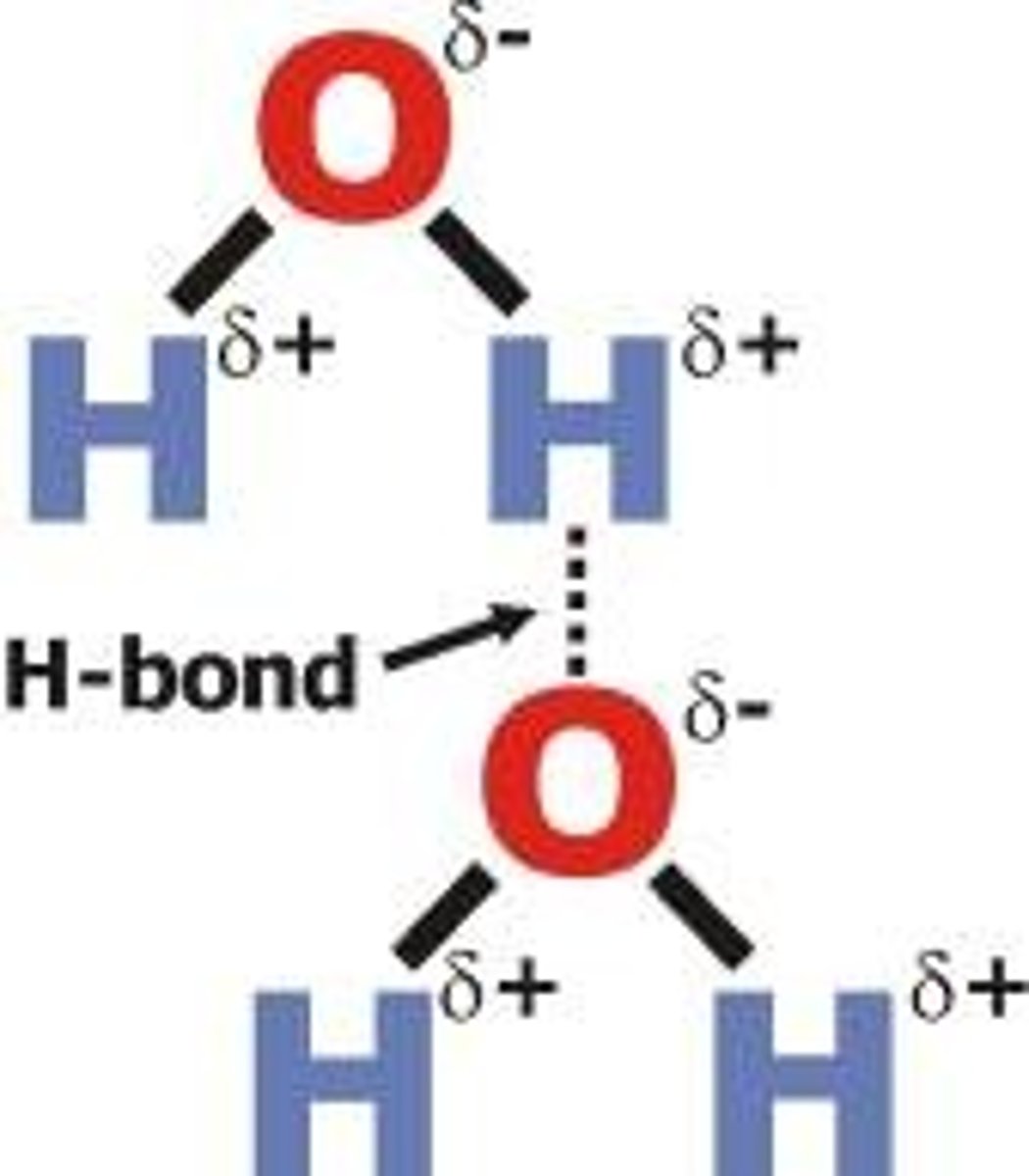
hydrogen bond
Attraction between a slightly positive hydrogen atom and a slightly negative atom.
Rank the intermolecular forces from weakest to strongest
London dispersion forces < dipole-dipole interactions < hydrogen bonds
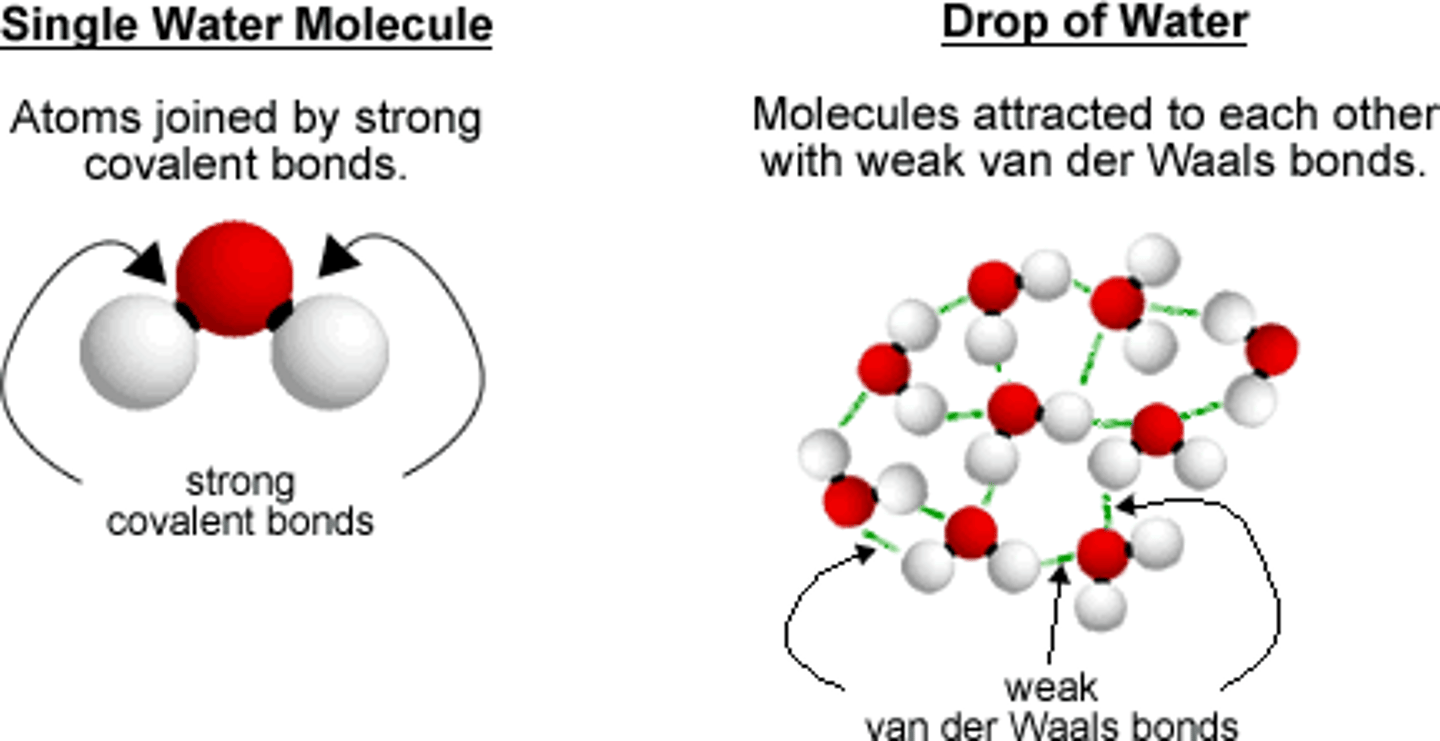
van der Waals forces
a slight attraction that develops between the oppositely charged regions of nearby molecules
melting point and boiling points between polar and nonpolar species
higher in polar due to dipole-dipole interactions.
Atoms that exhibit hydrogen bonding
hydrogen bonded to Fluorine, Oxygen, or Nitrogen.
Pick up the FON
Hydrogen bonds are a form of
dipole-dipole interactions that can be intra- or intermolecular. Not actually bonds. No sharing or transferring of electrons. H carries only a small amount of electron density when bonded to highly electronegative atoms. Leads to high boiling points.