The Periodic Table
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
What is the periodic table?
A table that displays all of the known elements
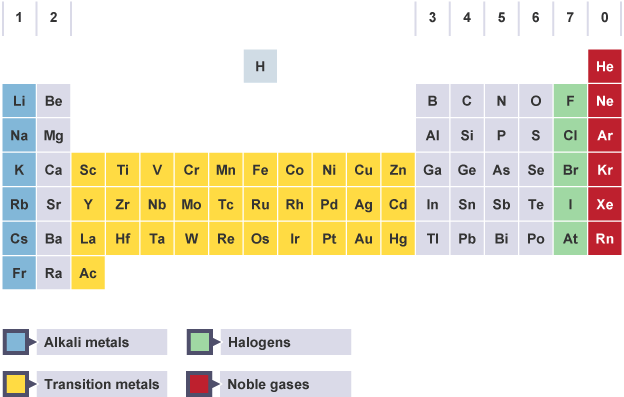
How are elements arranged in the periodic table?
In groups & periods by increasing atomic number
Groups are…
The vertical columns in the periodic table
The group number represents…
The number of electrons in the outer shell
Periods are…
The horizontal rows in the periodic table
The period number represents…
The number of electron shells
Why do elements in the same group have similar chemical properties?
Because they have the same number of electrons in the outer shell
Why are elements in group 8 / 0 less reactive?
Because they do not need to gain or lose any electrons as they already have a full outer shell
This makes them very stable
Electronic configuration is…
The number of electrons in each electron shell
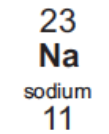
Electronic configuration of Na
2, 8, 1
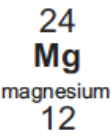
Electronic configuration of Mg
2, 8, 2

Electronic configuration of Al
2, 8, 3
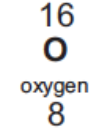
Electronic configuration of O
2, 6
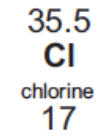
Electronic configuration of Cl
2, 8, 7

Electronic configuration of Ar
2, 8, 8
Where are metals & non-metals positioned on the periodic table?
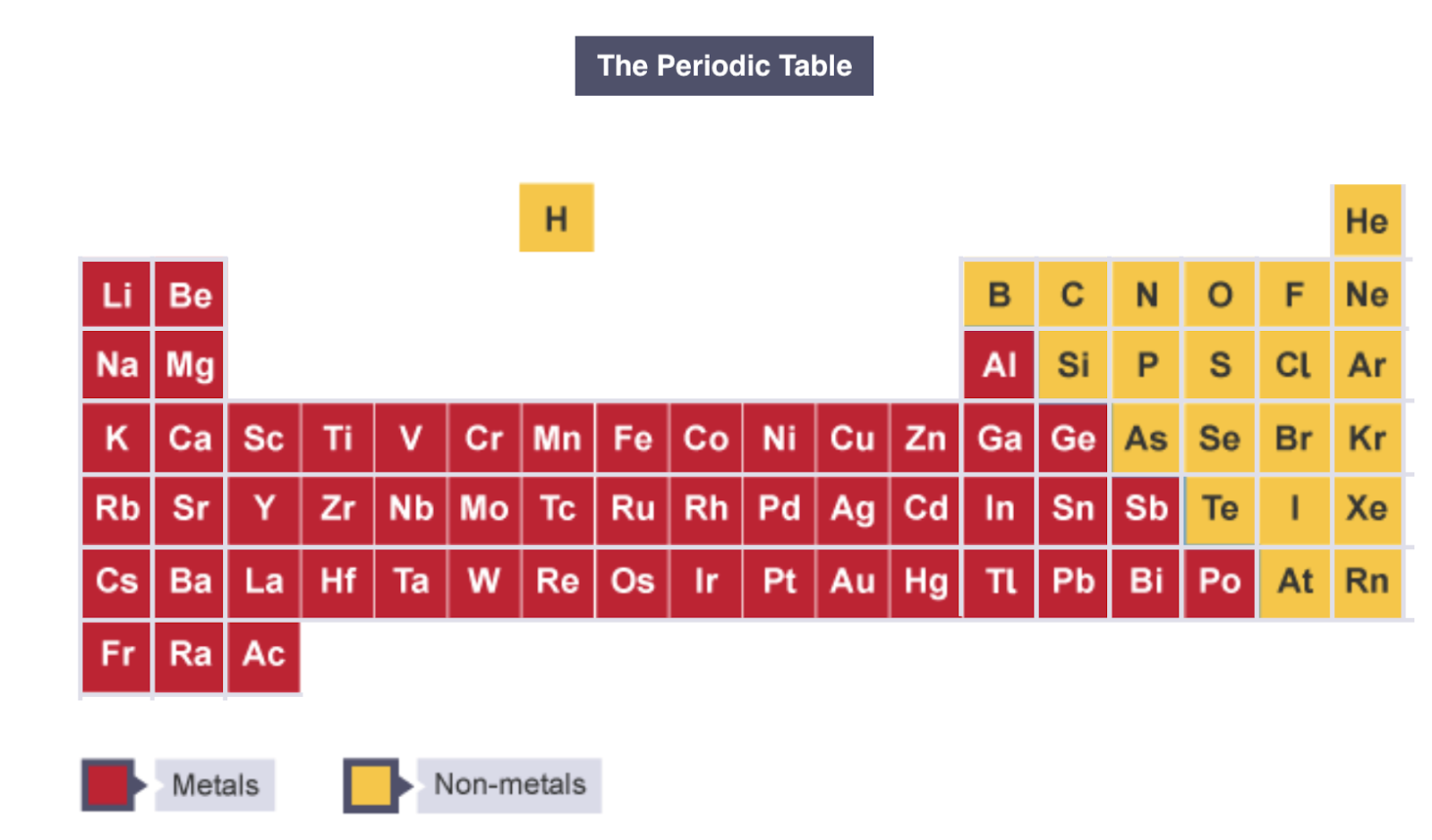
How can metals be identified without the periodic table?
Conductors of electricity
Form positive ions
Have basic oxides which react with acids to give water & a salt
How can non-metals be identified without the periodic table?
Not conductors of electricity (except graphite)
Form negative ions
Have acidic or neutral oxides