Lecture 5: Cell Birth & Cell Death
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
64 Terms
neoplasia
Irreversible proliferation of cells: proliferation continues even in the absence of an external stimulus
hashimoto disease (hypothyroidism)
apoptosis of thyroid cells (low T3/T4 levels)
fatigue, dry skin, “feeling down”
symptoms of Hashimoto disease
CPID, smokers (chronic damage to epithelial cells)
metaplasia clinical correlation
dysplasia of exocervix
Hashimoto disease
most common form of hypothyroid disease (opposite of Grave’s disease)
Fas ligand (when Fas receptor on lymphocytes bind to lignad, lymphocytes undergo rapid apoptosis)
at immunologically “privileged” sites (eye, testes), the vascular endothelial cells express
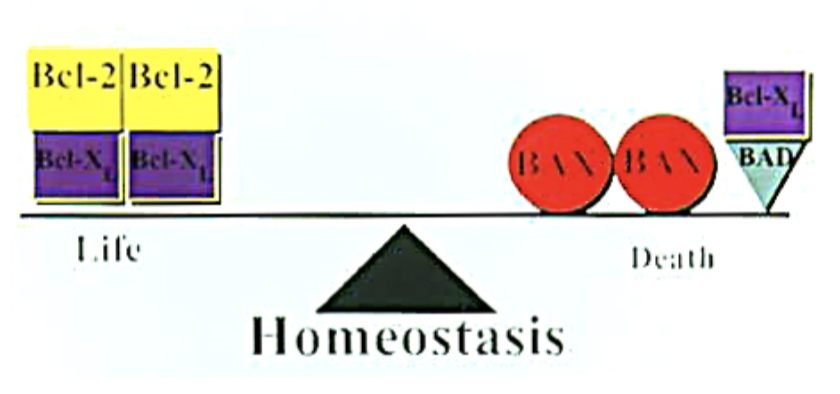
Bcl pro and anti-apoptotic
the ratio of what determines apoptosis in the mitochondria?
intrinsic pathway of apoptosis
Burkitt’s lymphoma reveals molecules and mechanisms for
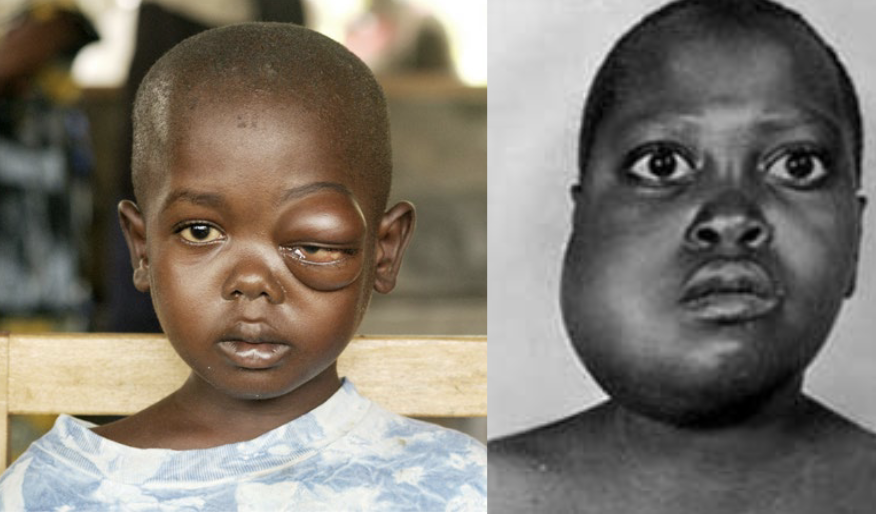
physiologic activators/inducers of apoptosis
TNF, FasL, growth/survival factor withdrawal, glucocorticoids
damage-related activators/inducers of apoptosis
viral infection, heat shock, toxins, tumor suppressors, oxidants/free radicals
therapy-associated activators/inducers of apoptosis?
UV/gamma irradiation, chemotherapeutic drugs
irreversible
is neoplasia reversible or irreversible?
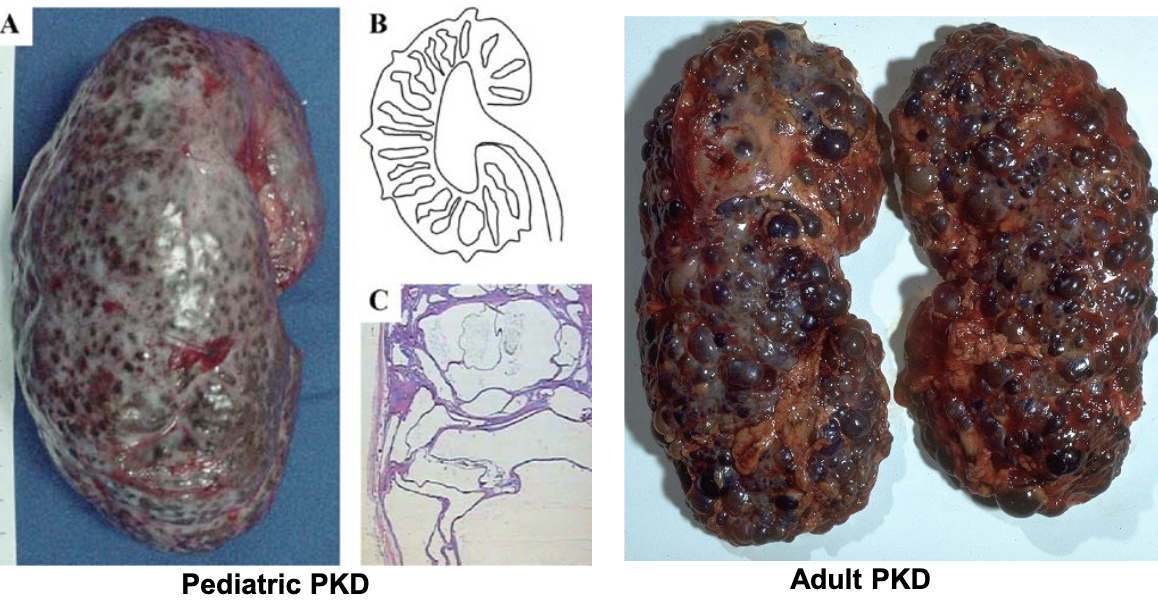
apoptosis (cysts on surface, large empty spaces)
polycystic kidney disease is an example of too much
reversible
is hyperplasia reversible or irreversible?
reversible
is metaplasia reversible or irreversible?
reversible
is dysplasia reversible or irreversible?
benign
malignant
what are the 2 major forms of neoplasia?
benign
loss of proliferation controls only (stays in place):
malignant
loss of both proliferation and positional controls
regeneration
1-for-1 replacement of lost cells by the same cell type
physiological (helpful)
regeneration is always pathological/physiological
regeneration
hyperplasia
metaplasia
dysplasia
what are 4 reversible altered proliferative states?
regeneration
endothelial regrowth following vascular surgery is an example of:
regeneration
liver regrowth after donating or receiving a transplant is an example of:
hyperplasia
increase in the number of cells in a tissue; cells are fully differentiated. Can be physiological (helpful) or pathological (harmful)
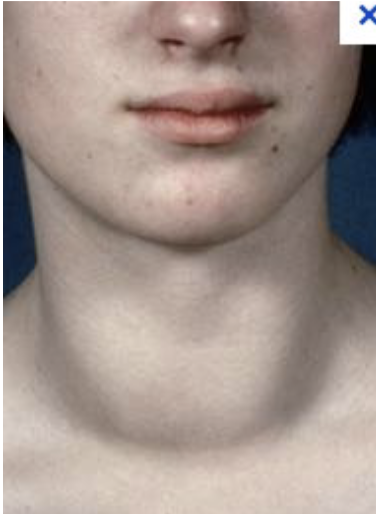
hyperplasia
Grave's disease (hyperthyroidism) is an example of thyroid _________
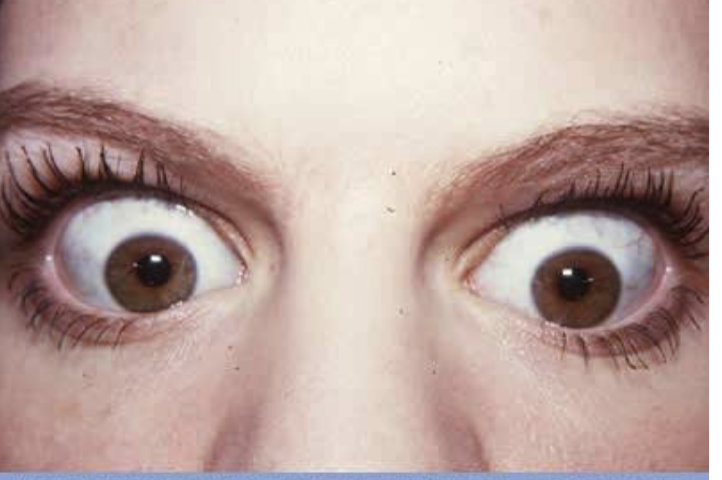
hyperplasia (helpful version)
an increase in hematopoietic cells in bone marrow following severe blood loss or changes in altitude is an example of ______
hyperplasia
an increase in smooth muscle cells in the arterial wall in atherosclerosis or following vascular surgery (restenosis) is an example of ________
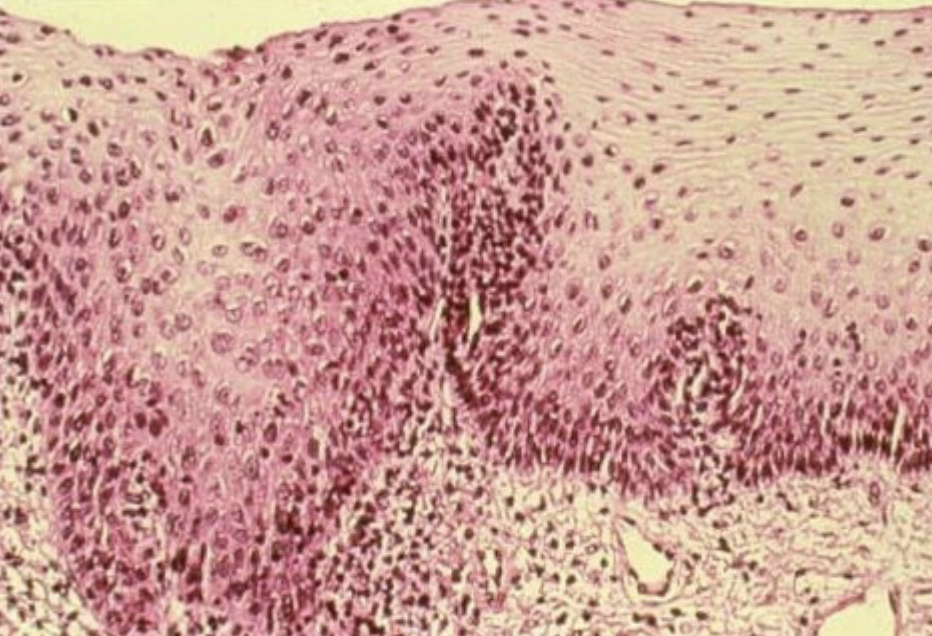
metaplasia
adaptive substitution of one cell type for another
metaplasia
Replacement of ciliated columnar epithelium by stratified squamous epithelium in response to chronic inflammation (eg. chronic pelvic inflammatory disease or smoking) is an example of ________
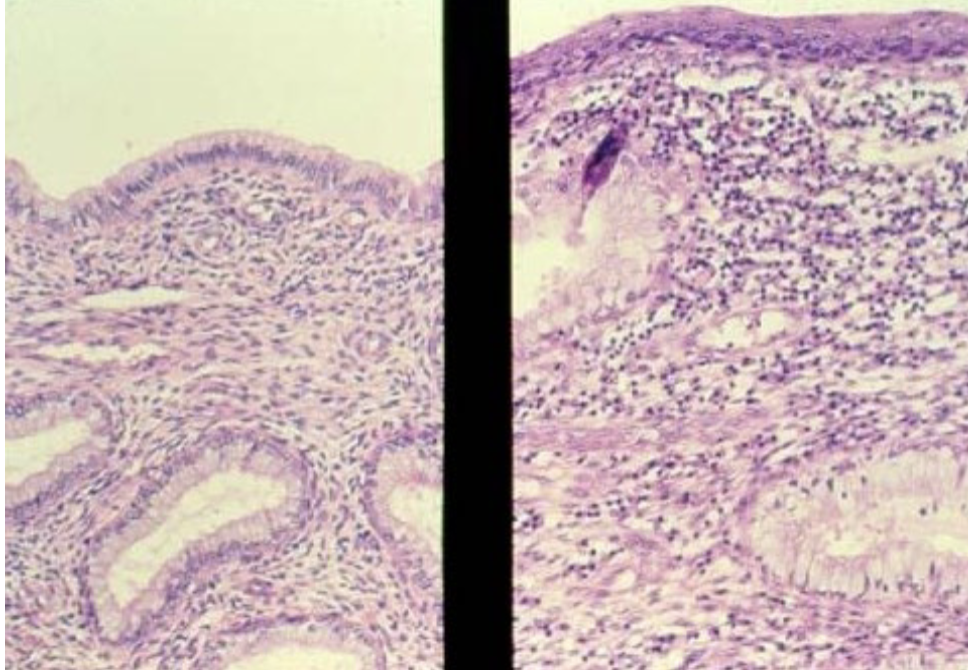
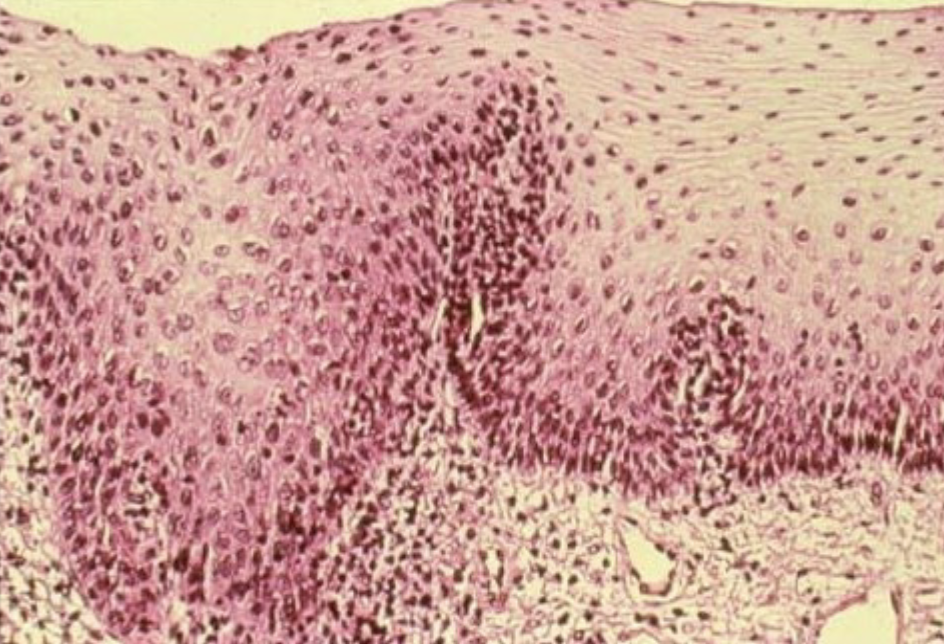
dysplasia
activated metabolic pathways for proliferation; loss of orientation in a tissue. Abnormal appearance of cells
cancer
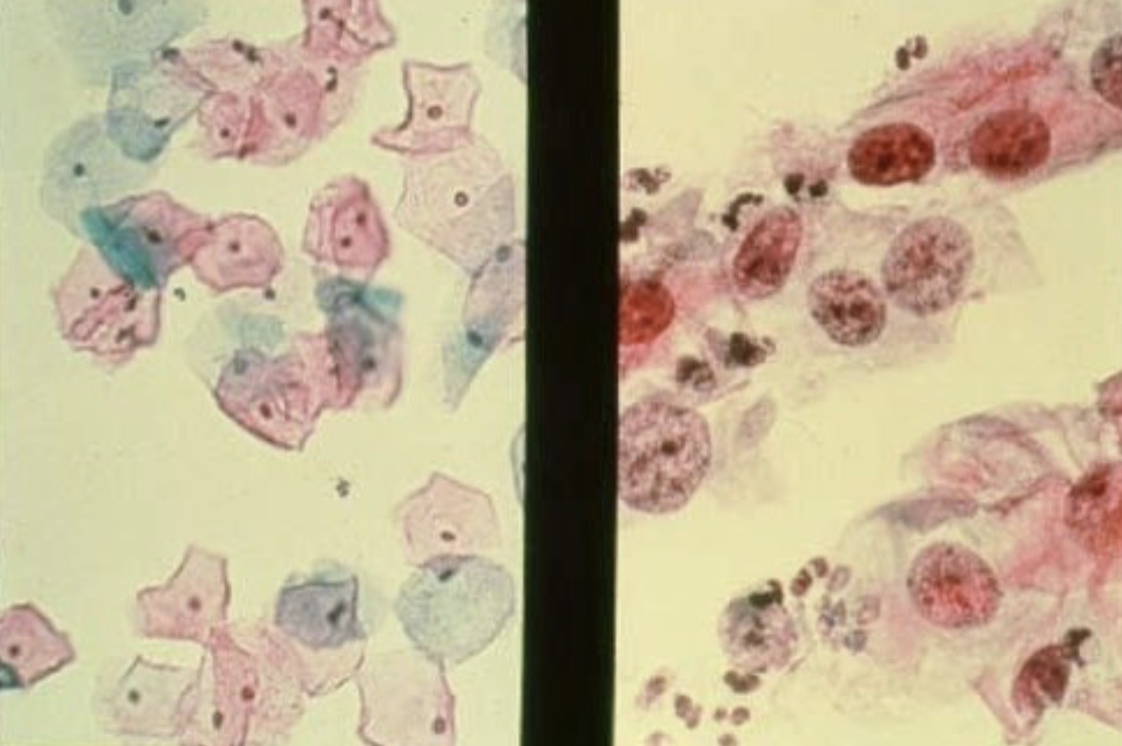
cervical dysplasia as seen on a Pap Smear in women may be an early sign of _______
“benign”
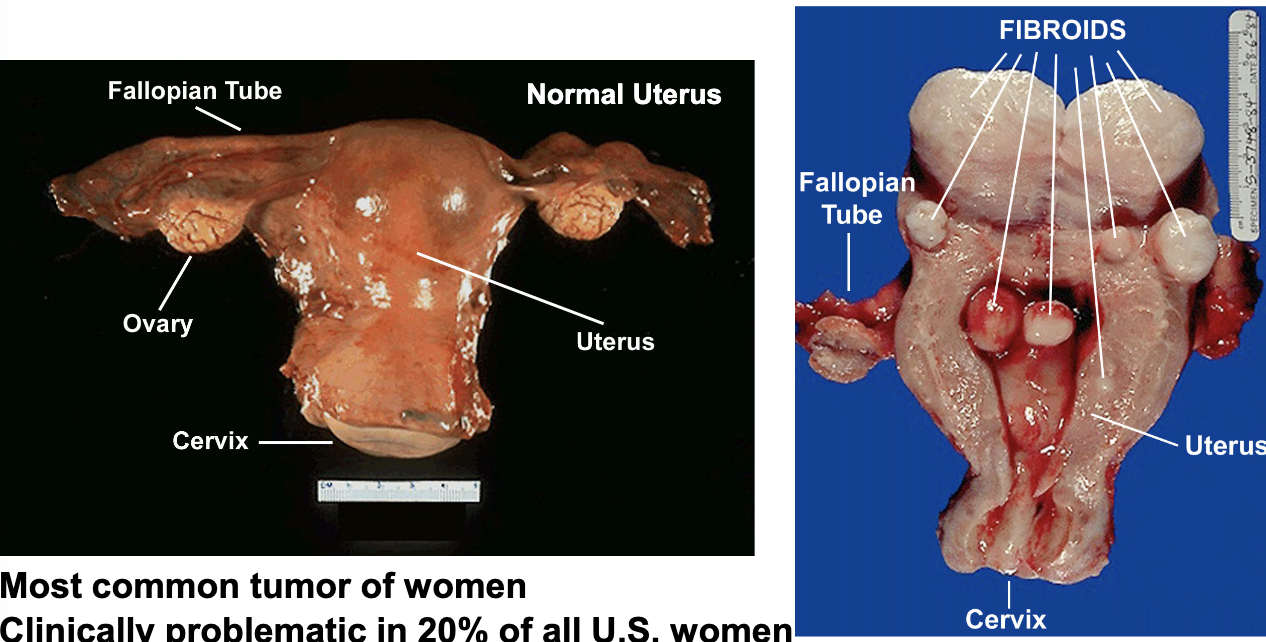
uterine fibroids are a ________ neoplasia of smooth muscle cells. most common tumor of women (affects black women disproportionately)
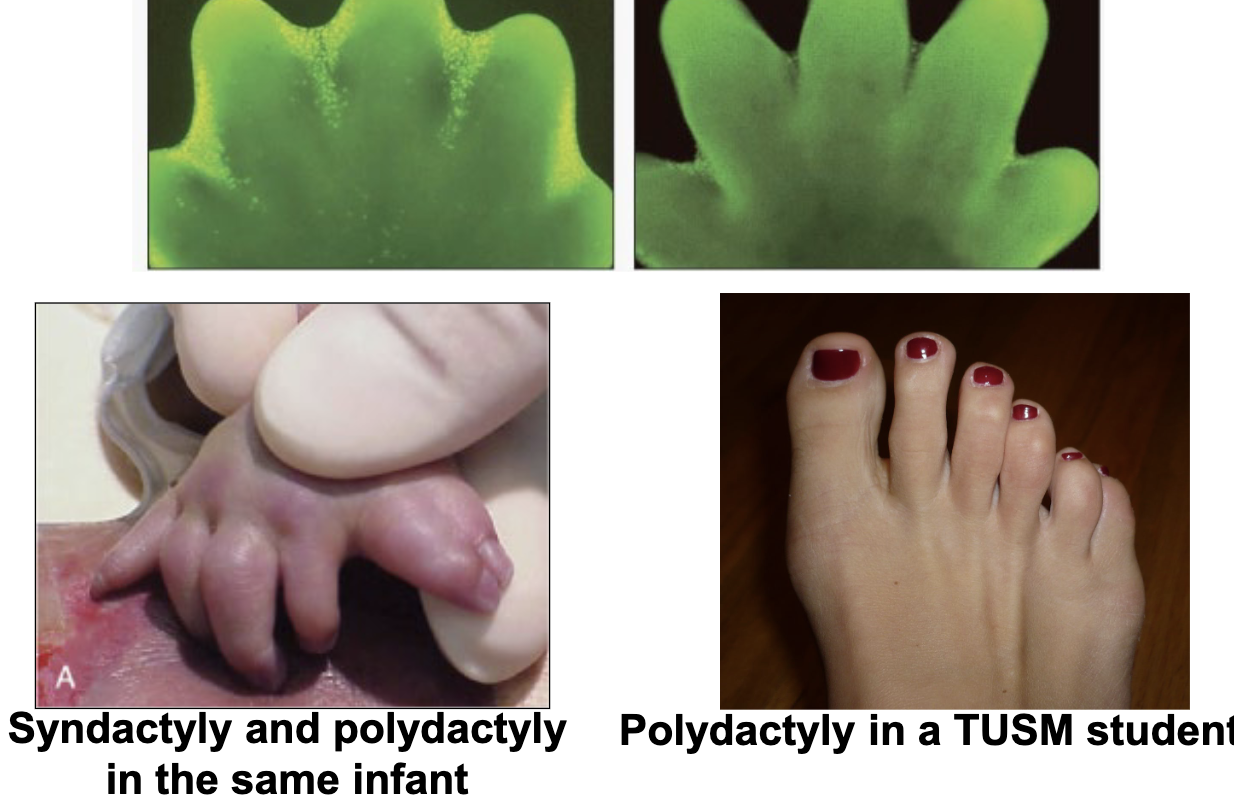
apoptosis
what cellular even has a critical role in determining the presence of syndactyly/polydactyly or not?
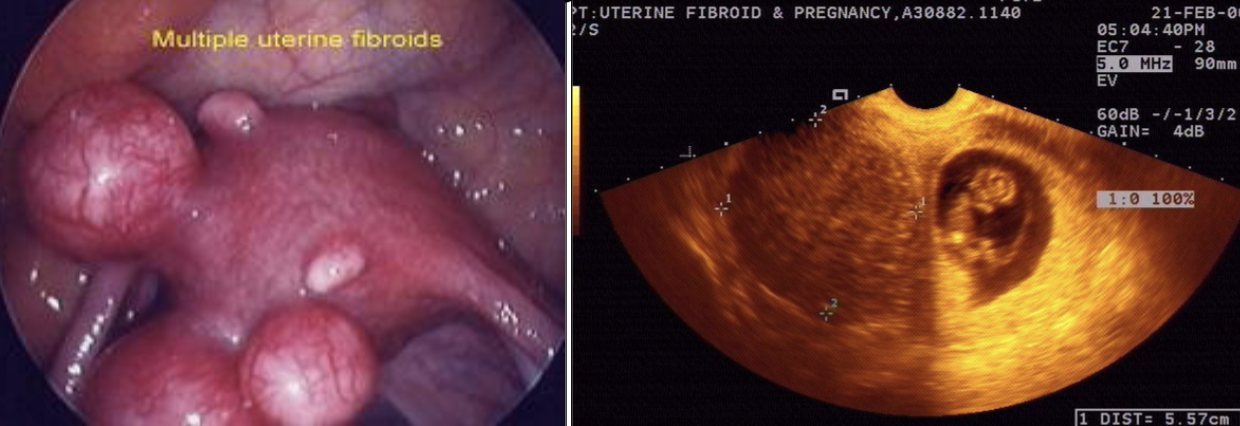
uterine fibroids
symptoms: abnormal, heavy bleeding; pain/pressure; fertility problems
apoptosis
polysistic kidney disease occurs when there is too much ________
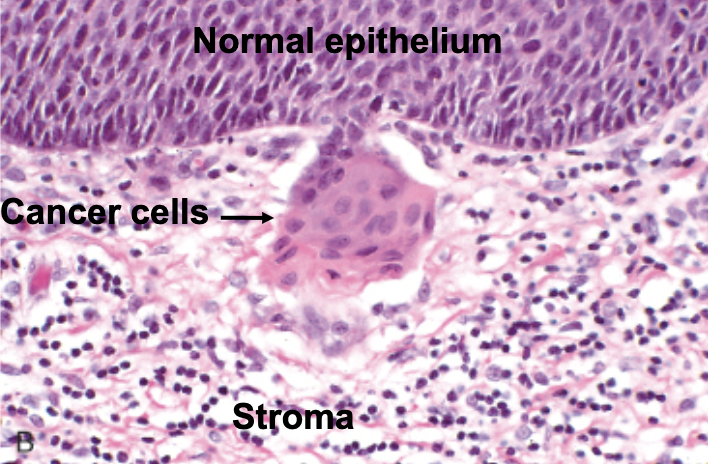
malignant (crossing the boundary)
cancer = ____________ neoplasia
critical for maintaining homeostasis (cell proliferation = cell death)
why have apoptosis?
apoptosis
tissues with greatest frequencies of cell proliferation also exhibit the greatest frequency of _________, including thymus, spleen, small intestine, epidermis, ovarian follicles
necrosis
________ is triggered by sustained ischemia, physical, or chemical trauma.
cells swell, organelles damaged, chromatin randomly degraded
inflammation (cells lyse, organelles destroyed)
necrosis causes
apoptosis
_________ is triggered by specific signals that activate specific genes. cells shrink, organelles intact, chromatin degraded systematically.
phagocytosis (membrane blebs, cell contents retained)
apoptosis causes
induction
modulation
execution
what are the 3 phases of apoptosis?
intrinsic pathway of apoptosis
the modulation step of apoptosis only exists in which pathway?
Bcl proteins
the modulation phase of apoptosis is regulated by:
extrinsic pathway of apoptosis
TNF-α is an activator for which pathway?
extrinsic pathway of apoptosis
Fas is an activator for which pathway?
extrinsic pathway of apoptosis
in which pathway of apoptosis do proteins bind to a cell receptor that directly activates caspase cascade?
intrinsic pathway of apoptosis
growth/survival factor withdrawal is an activator for which pathway?
intrinsic pathway of apoptosis
a viral infection is an activator for which pathway?
intrinsic pathway of apoptosis
heat shock is an activator for which pathway?
intrinsic pathway of apoptosis
toxins and free radicals are activators for which pathway?
intrinsic pathway of apoptosis
UV/gamma irradiation and chemotherapeutic drugs are activators for which pathway?
executioner caspases
caspases followed by endonucleases; caspases are directly responsible for blebbing:
mitochondria
where are all the Bcl proteins located?
cytochrome c
what molecule comes out of mitochondrial channels during the intrinsic apoptosis pathway that will initiate executioner caspases?
bcl proteins
If the apoptotic signal utilizes the intrinsic (mitochondrial) pathway, the process may be modulated (either enhanced or dampened) by:
extrinsic pathway of apoptosis
death receptors activating the caspase cascade is a feature of which pathway?
intrinsic pathway of apoptosis
which pathways can be modulated either up (pro-apoptotic) or down (anti-apoptotic) by the Bcl family proteins?
caspases followed by endonucleases
the chief executioners of the cell in both the intrinsic and extrinsic pathways for apoptosis:
FAS receptor (undergo rapid apoptosis upon entering priveleged sites)
immunologic immunity ("privilege") is conferred on sites like the eye and testis because immune cells (lymphocytes) constitutively express:
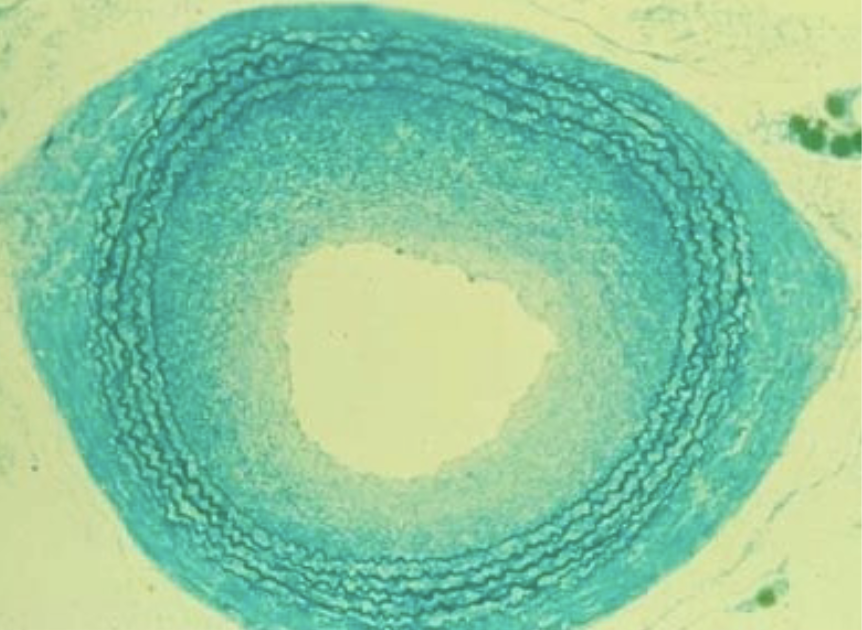
restenosis (smooth muscle cell hyperplasia) following balloon angioplasty
what is this depicting?