Chem 1411 - Chapter 3 - Percent Composition and Empirical Formula & Combustion Analysis
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Percent (by mass)
What percentage of the element's mass makes up the compound's total mass?
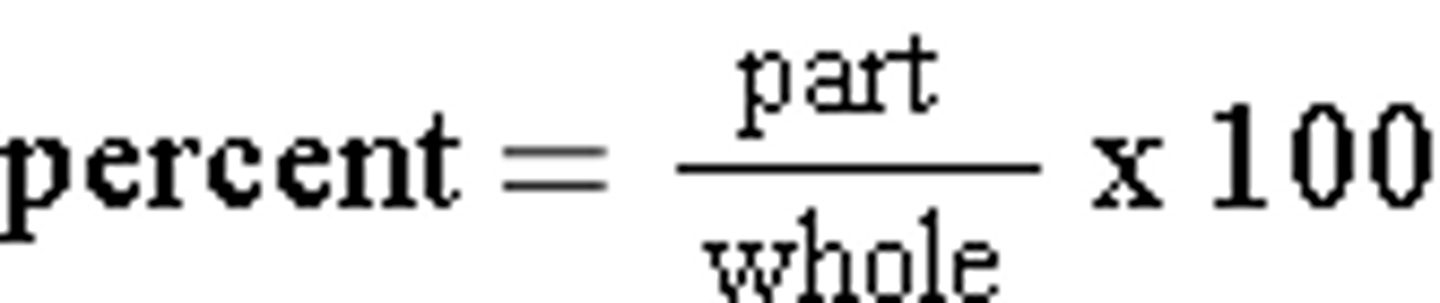
Percent (by mass) Worked out
In this case, the part is the element and the whole is the compound. From our subscript formula, we can find this relationship. For example with glucose, we know that
Since we are finding the percentage by mass we will need to convert the moles to mass and then multiply by 100 to get the percentage. Let's find the percentage by mass of the H. First we will convert the moles to grams using what conversion factor?
We will use our molar mass conversion factor which we can find using the periodic table.
12 moles H x 1.01 g/mol = 12.12 g H
1 mole C6H12O6 x 180.0 g/mol = 180.0 g C6H12O6
We can now find the percentage by mass for the hydrogen.
See Next Difinition
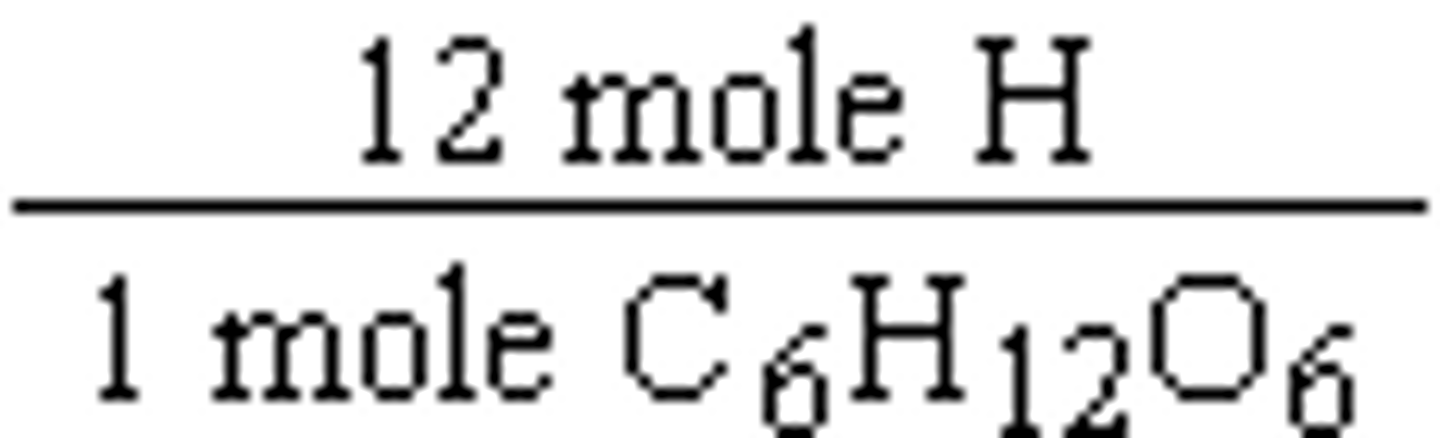
Percent (by mass) Worked out Cont.
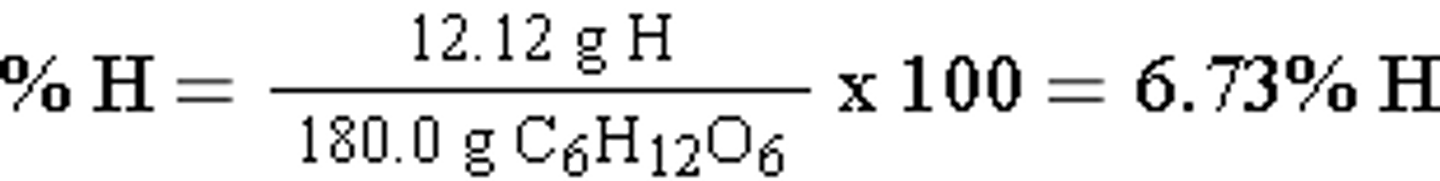
Steps to Finding Empirical Formulas
1. Obtain the amount in grams of each element.
2. Convert the grams of each element into moles.
3. Find the moles in whole numbers (subscripts are not in decimals).
4. Write the empirical formula.
Molecular Formula
In summary, to find the molecular formula from the empirical formula, we follow these steps:
1. Calculate the molar mass of the empirical formula
2. Divide the molar mass of the molecular formula by the molar mass of the empirical formula to obtain the factor
3. Multiply the empirical formula by the factor to obtain the molecular formula.
To find the molecular formula we will need to use molar mass. The molar mass of the compound is given to us in the problem, and we can find the molar mass of the empirical formula.
We will use these numbers to determine the molecular formula.
We recall that the molecular formula = empirical formula x factor
Some examples are: C2H2 = CH x 2
C6H6 = CH x 6
C5H10O5 = CH2O x 5
For example, the empirical formula of butanedione was determined to be C2H3O and its molar mass is 86.09 g/mol. What is the molecular formula?
The molar mass of the molecule is given to us. We calculate the molar mass of the empirical formula to be
2(12.01 g/mol) + 3(1.01 g/mol) + 16.00 g/mol = 43.04 g/mol
Once we have the factor, we can determine the molecular formula.
molecular formula = empirical formula x factor
molecular formula = C2H3O x 2
molecular formula = C4H6O2
The Formula Is Reversed... MAKE SURE TO PUT REVERSED.
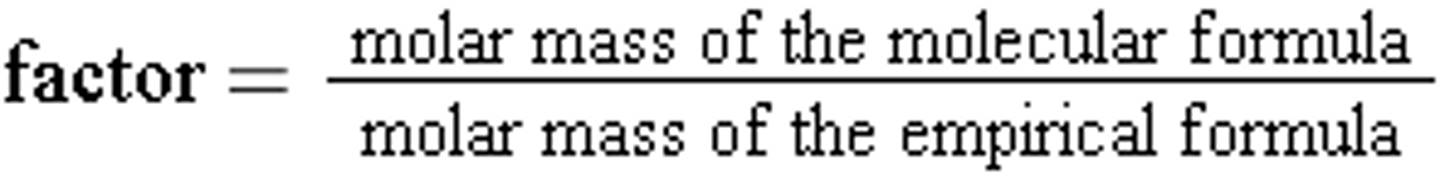
Combustion Analysis
To determine the empirical formula, we will need the moles of C from the g of CO2 and the moles of H from the g of H2O. This is what we have done before.
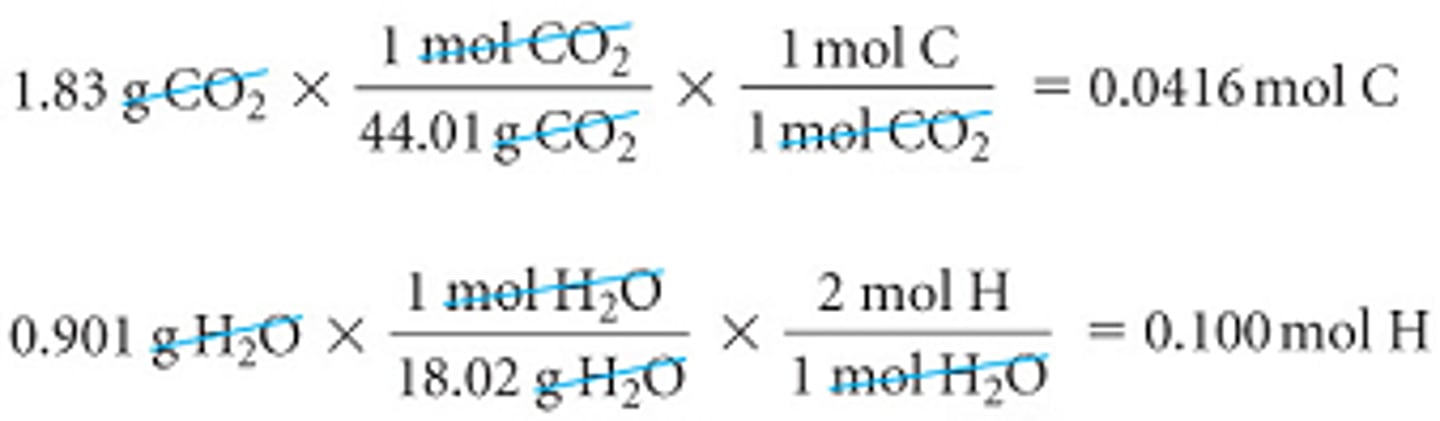
Combustion Analysis Cont. 1
Once we have the moles of each element, recall that we divide by the smallest number which would be 0.0416.
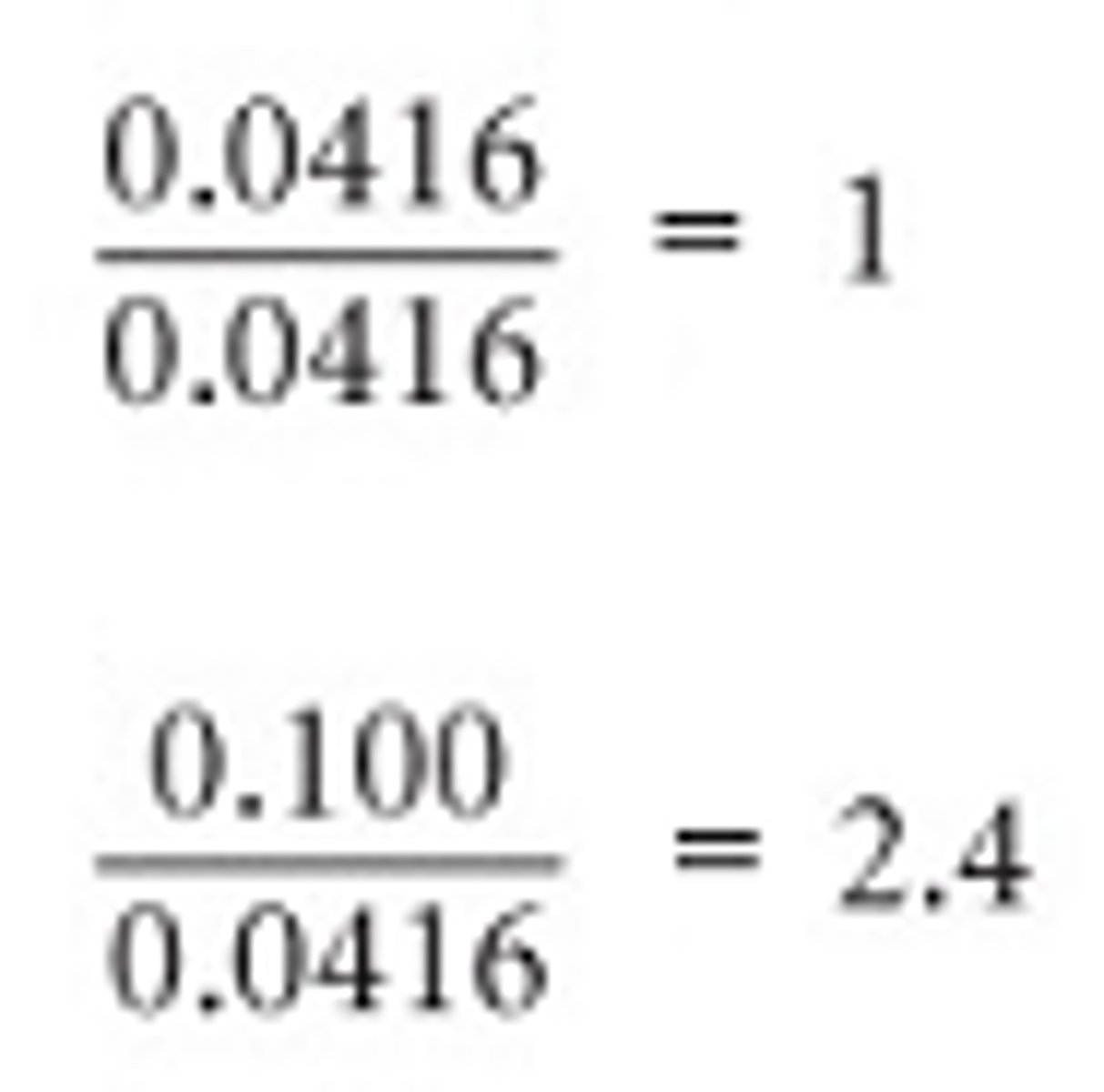
Combustion Analysis Cont. 2
However, we still do not have the smallest whole number ratio. We will need to multiply by a whole number to obtain whole number ratios.
So our empirical formula is C5H12.
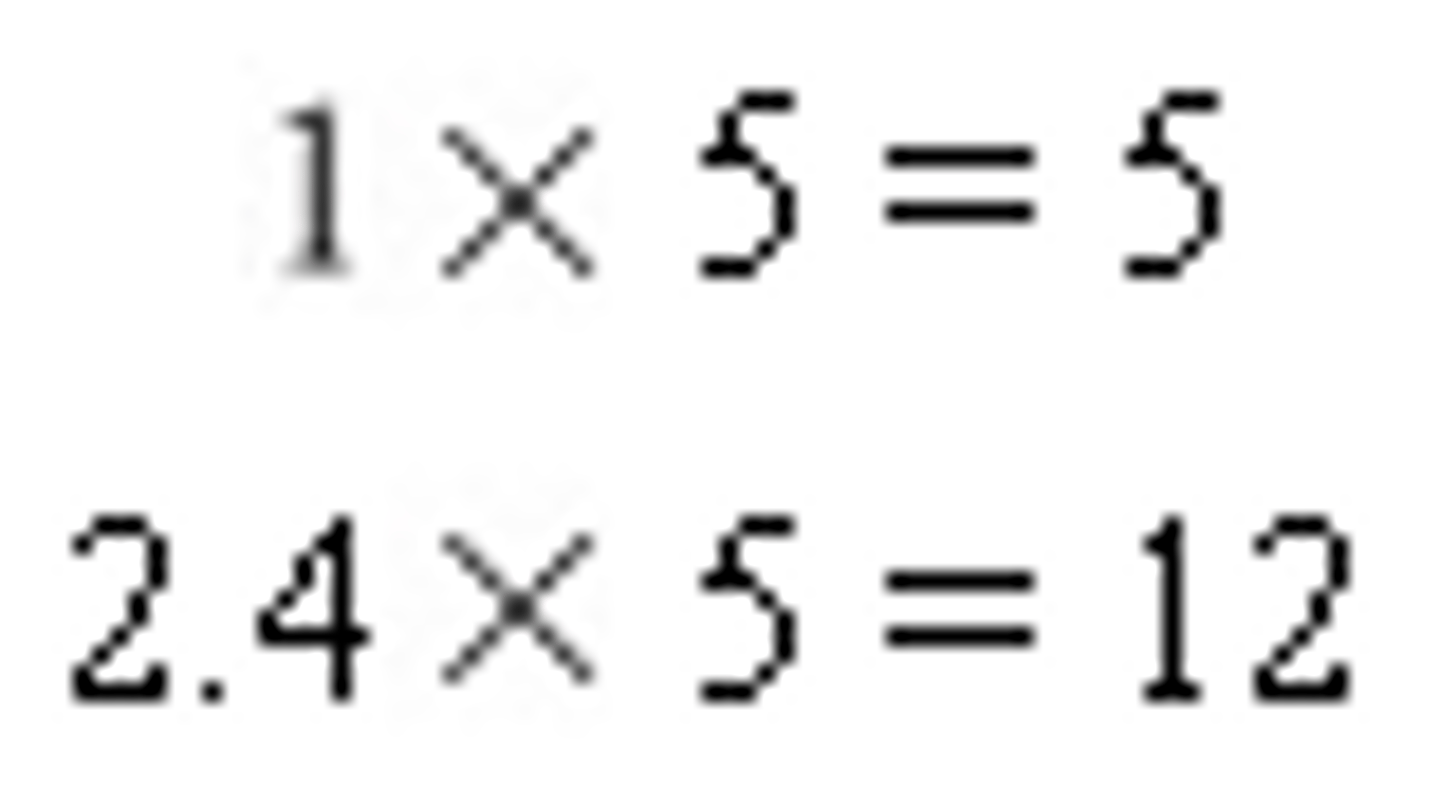
Combustion Analysis #2
For example, upon combustion a 0.8233 g sample of a compound containing carbon, hydrogen, and oxygen produced 2.445 g of CO2 and 0.6003 g of H2O.
Since we are to determine the g of oxygen by subtracting the masses of C and H from the compound's starting mass, we will need the g of carbon and the g of hydrogen instead of the moles of carbon and the moles of hydrogen as in our previous example.
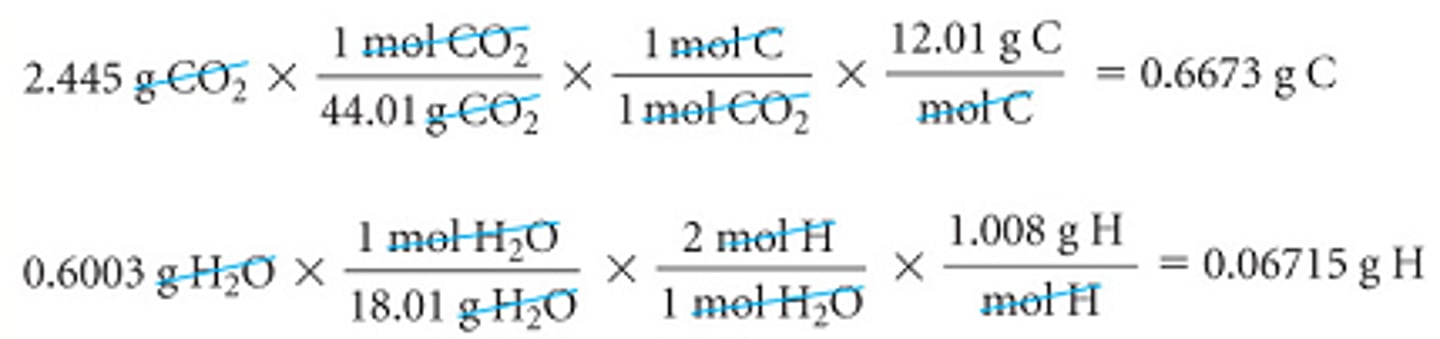
Combustion Analysis #2 Cont.
The total mass of the C, H and O must equal the compound's starting mass which was 0.8233 g.
Therefore, the mass of the O = mass of compound - (mass of C + mass of H)
We now have the g of C, g of H, and the g of O. From here we can find the empirical formula.

Combustion Analysis #3
Menthol, the substance we can smell in mentholated cough drops, is composed of C, H, and O. A 0.1508 −mg sample of menthol is combusted, producing 0.4244 mg of CO2 and 0.1739 mg of H2O. What is the empirical formula for menthol?
work out the moles and mass of C in the sample from the mass of CO2 produced... All the C from the menthol sample ends up in the CO2
mass CO2 = 0.422 mg / 44.01 g/mol = 0.009645 mmol
mmoles C = 0.009645 mmol
mass C = 12 g/mol x 0.009645 mol = 0.1157 mg
work out moles and mass of H from the mass of H2O produced
mmoles H2O = 0.1739 mg / 18.02 g/mol = 0.00965 mmol
mmoles H = 2 x mmoles H2O = 0.01930 mg
mass H = 1.01 g/mol x 0.01930 mmol = 0.01949 mg
Now, mass O in the sample = total mass - mass C - mass H
mass O = 0.1508 mg - 0.1157 mg - 0.01949 mg = 0.01561 mg
mmol O = 0.01561 mg / 16.00 g/mol = 0.0009756 mmol
ratio moles C : H : O
= 0.00965 : 0.01930 : 0.0009756
divide each number by the smallest number
0.00965 / 0.0009756 : 0.01930 / 0.0009756 : 0.0009756 / 0.0009756
= 10 : 20 : 1
C10H20O