Geology 1121- Quiz 2 (Ch.3)
1/25
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
What are minerals made from?
bonded atoms
What is mineralogy?
the study of minerals
What is a mineralogist?
a person who studies minerals
What are the 6 characteristics of a mineral?
naturally occurring
formed by geological processes
solid
crystalline structure
definable chemical composition
generally inorganic
What is an atom?
the smallest unit of an element that can combine in chemical reactions
Which part of the atom has the most mass?
nucleus
How do you calculate atomic number?
count the protons in the atom
How do you calculate atomic mass?
add the number of protons and neutrons together
What is an isotope?
an atom of the same element has a different numbers of neutrons
How do minerals form from chemical reactions?
solidification of a melt
precipitation from water
solid-state diffusion
biomineralization
precipitation from gas
What type of bond does electron sharing produce?
covalent
What is the strongest mineral we know of?
diamond
What does Mohs hardness scale measure?
the hardness of a mineral
What type of bond does electron transfer create?
ionic (weak)
What is the most common type of bond?
ionic
What type of minerals do ionic bonds create?
weak minerals
How are minerals grouped?
chemical class and composition
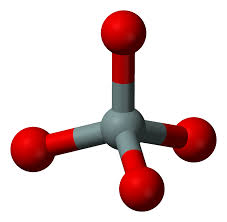
What chemical class looks like this?
silicates (center is silicon and outside is oxygen)
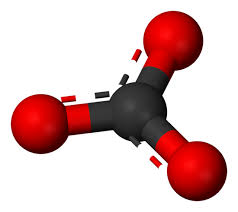
What chemical class looks like this?
carbonates (center is carbon and outside is oxygen)
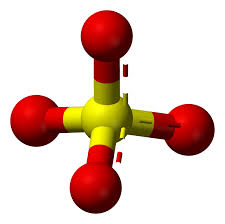
What chemical class looks like this?
sulfates (center is sulfur and outside is oxygen)
What type of bonding is present in silicate bonds?
covalent and ionic, electron sharing and transfer
What are the most abundant silicates?
feldspars
What type of bonding occurs in carbonates?
ionic, electron transfer
What type of bonding occurs in oxides?
ionic, electron transfer
How do most sulfates form?
precipitation
What are the 3 characteristics of halides?
naturally occurring
electronically neutral
masses of the same element