Gen Chem MCAT
1/14
Earn XP
Description and Tags
The Periodic Table
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Lithium and sodium have similar chemical properties. For example,both can form ionic bonds with chloride. Which of the following best explains this similarity?
Lithium and sodium are in the same group of the Periodic Table and have the same number of valence electrons, which leads to similar reactivity and bonding characteristics. The alkali metals, Group 1, are characterized by having one electron in their outermost shell.
Carbon and silicon are the basis of biological life and synthetic computing, repectively. While these elements share many chemical properties , which of the following bst describes a difference betweeen the two elements?
Carbon has a smaller atomic radius than silicon. This leads to stronger covalent bonds in carbon.
What determines the length of an element’s atomic radius?
The number of valence electrons
The number of electron shells
The number of neutrons in the nucleus.
1 and 2 only
Ionization energy contributes to an atom’s chemical reactivity. Which of the following shows an accurate ranking of ionization energies from lowest to highest?
first ionization energy Li> first ionzation energy of Be> second ionization energy of Be
Antimony is used in some antiparasitic medications—specifically those targeting Leishmania donovani. What type of element is antimony?
Metalloid.

The properties of atoms can be predicted, to some extent, by their location within the periodic table. Which property or properties increase in the direction of the arrows shown?
Electronegativity
Atomic radius
First ionization energy
1 and 3 only
Metals are often used for making wires that conduct electricity. Which of the following properties of metals explains why?
Metals have valence electrons that can move freely.
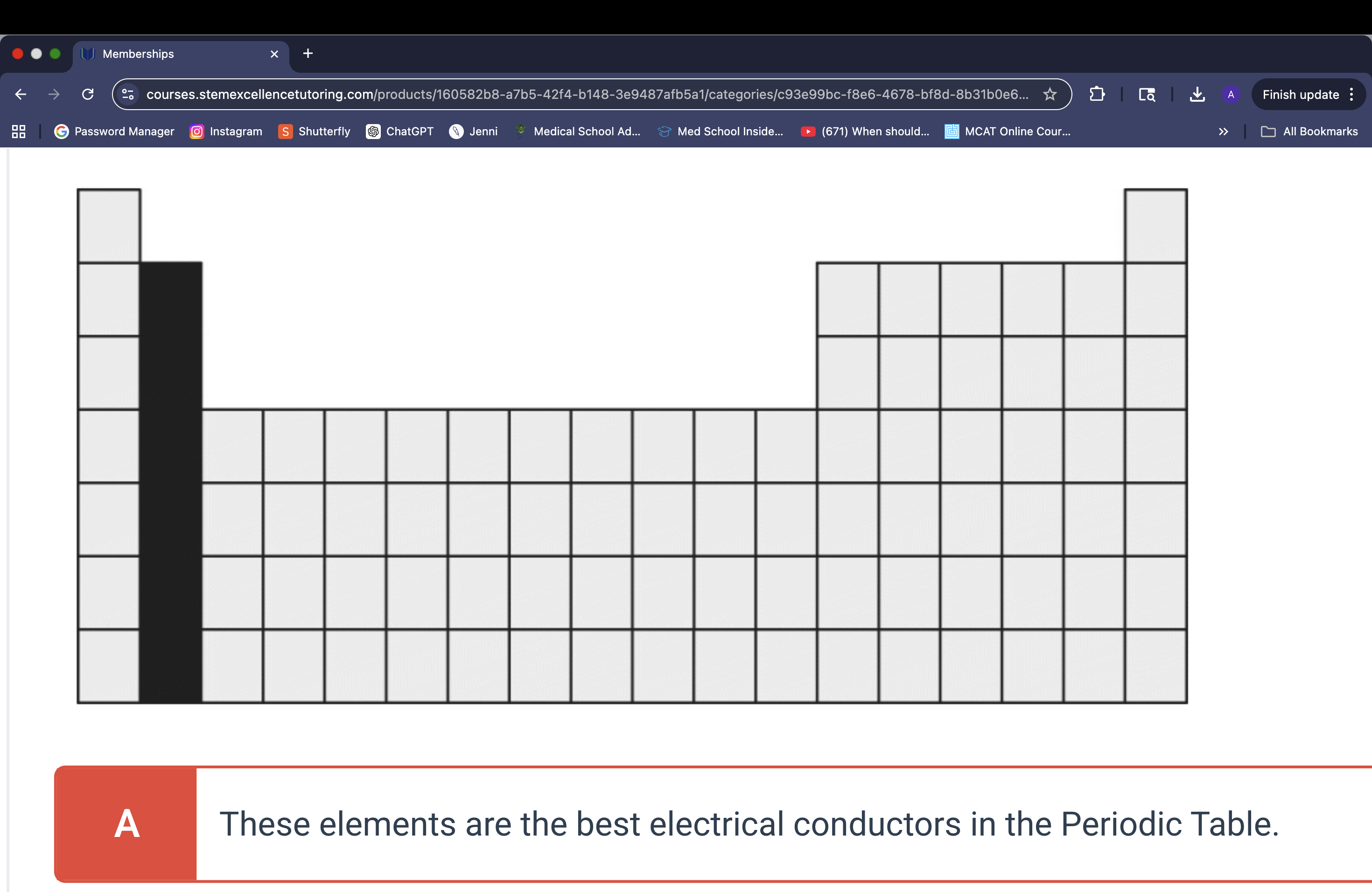
Which of the following is an important property of the group of elements shaded in the Periodic Table below?
These elements form divalent cations.
When dissolved in water, which of the following ions is mot likely to form a complex ion with H2O?
Fe2+
How many valence electrons are present in elements in the third period?
The number increases as the atomic number increases.
Which of the following elements has the highest electronegativity?
Cl
Of the four atoms depicted here, which has the highest electron affinity?
Seven valence electrons and balanced equally in 2 p orbitals, making it very likely to gain an additional electron.
Which of the following atoms or ions has the largest effective nuclear charge?
K+
Why do halogens often form ionic bonds with alkaline earth metals?
The halogens have much higher electron affinity compared to alkaline earth metals, allowing them to attract and gain electrons, resulting in ionic bonds.
What is the highest-energy orbital of elements with electrons in the n=3 shell?
d-orbital