IMMU - Chapter 6 The Development of B Lymphocytes
1/92
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
93 Terms
Outcome of B cell development
Generation of B cells with diverse array of BCR
B cells capable of recognizing foreign antigen
Selection of B Cells with no reactivity to self-antigen (Positive selection)
Removal of B cells with strong reactivity to self-antigens (Negative selection)
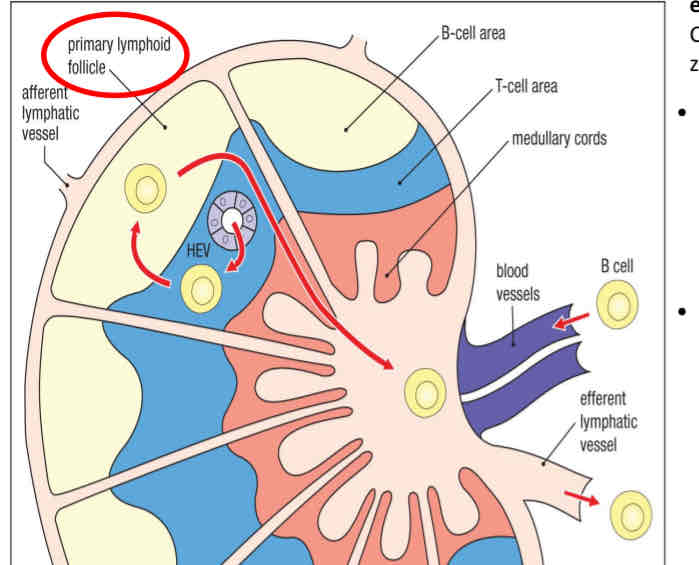
Lymphoid tissues
Organized structure that support immune responses
Central/primary
Sites of lymphocyte development and maturation
Bone marrow - B cells
Thymus - T cells
Peripheral/Secondary
Sites of lymphocyte activation and differentiation into effector cell types
spleen, lymph nodes, adenoids, tonsils, mucosal tisssue
Tertiary lymphoid tissue
Chronic inflammation can lead to the development of tertiary lymphoid tissue
Bone niche
B cells develop in the bone marrow and migrate to peripheral lymphoid organs, where they can be activated by antigens
B cell development occurs in..
Bone marrow
B cell activation starts when
The B cell meets an antigen in the lymph node
Bone marrow microenvironment is composed of
a cellular compartment
An extracellular matrix
A liquid compartment
A liquid compartment
A mixture of growth factors, cytokines
Extracellular matrix
A complex network of matrix proteins
Cellular compartment
Cells of hematopoietic and non-hematopoietic origin and effector immune cells
Bone marrow stromal cells
A specialized network of non-lymphoid connective tissue that provide structural and physiological support to hematopoietic cells
Role of bone marrow enviornment
provide cell-cell contact (structural support) for developing B cells and other immune cells
Provide signals through cytokines for growth, survival and maintenance → IL-7 and CXCL12
What is notch?
a cell surface receptor that interacts with a transmembrane
Four different receptors
Notch1 signaling - promotes T-cell differentiation from common lymphoid progenitor
IL-7 promotes the
survival
Proliferation
And maturation of developing lymphocytes
The early stages of B-cell development are dependent on..
Bone marrow stromal cells
B-cell lineage
Notch 1 signal absent
Early IL-7R expression
Early T cell Progenitor
Notch 1 signal present
No early IL-7R expression
ILCs
Notch 1 signal present
IL-7R not required
Different transcription factor expression
Cells at different stages of development are identified by
Different combinations of CD proteins on their surface
CD34 are present on all HSC
CD127 is IL7Ra
CD19 is Pan B cell marker - all B cells have it
Checkpoint
molecular mechanism by with the B cell determines if the preceding step has been successfully completed
1st checkpoint - did developing B cell successfully express new IgH as pre-BCR on cell surface
2nd checkpoint - is the B-cell self reactive?
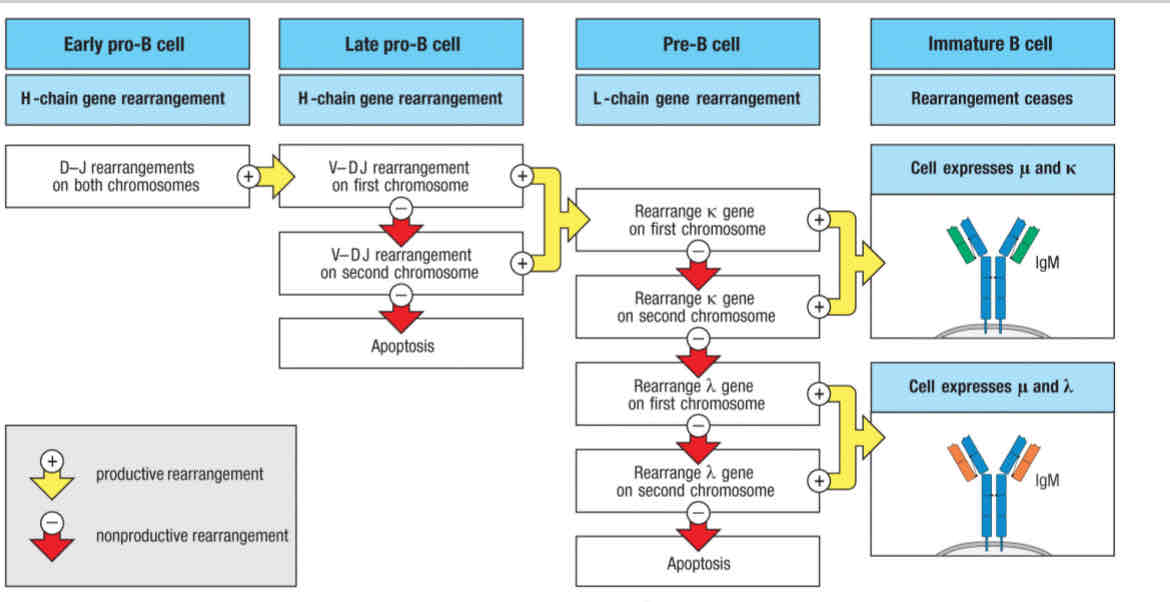
Step 1 - Heavy Chain rearrangement and
1st checkpoint
Step 2- Light chain rearrangement
2nd checkpoint
Stages of B cell development
Early pro-B cell
Late pro-B cell
Large pre-B cell
Small pre-B cell
Immature B cell
Mature B cell
Cell proliferation
Increasing the number of cells
Cell differentiation
Differentiating into another type of cell
Early pro-B cell and Large pre-B cell
Does cell proliferation
(Increasing the number of cells)
What determines this sequence?
RSS sequence dictates the order of rearrangements
Enhancers and promoters initiate transcription in a sequence and linked manner
Rearrangement and transcription of immunoglobulin genes are linked processes
In HSCs, Ig Heavy-chain locus is closes
In pro-B cells, B cell-specific transcription factors bind Ig enhancers and promoters
In late pro-B cell, DJ rearrangement occurs
In large B-cell, V-DJ rearrangement occurs
B cell lineage occurs when:
Notch 1 signal is absent
Early IL-7R expression
Early T cell progenitor occurs when:
Notch 1 signal present
NO Early IL-7R expression
ILCs (innate-like cells) occurs when:
Notch 1 signal present
IL-7R not required
Different transcription factor expression
Does the rearrangement give rise to a fully function heavy chain?
If no:
The other chromosome rearranges
Does the rearrangement give rise to a fully function heavy chain?
The rearrangement is then tested
If it fails, to produce a productive rearrangement
The developing B cell dies by apoptosis
Option 1: B cell maturation is coupled to immunoglobulin rearrangement
Option 1: B cell maturation is coupled to immunoglobulin rearrangement
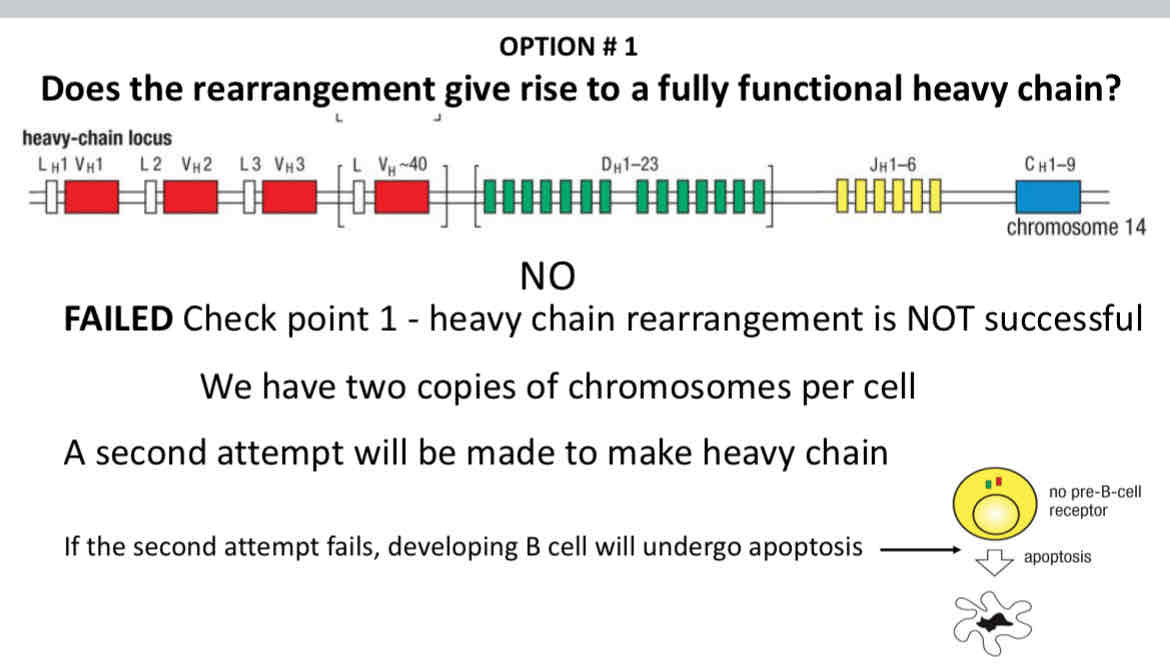
Only one productive rearrangement of the heavy chain is allowed, which is called
Allelic exclusion
Allelic exclusion at the immunoglobulin loci gives rise to B cells have antigen receptors of:
Monospecifity (single specifity)
Apoptosis
Used by immune system for deletion of immune cells
does not induce any inflammatory reaction
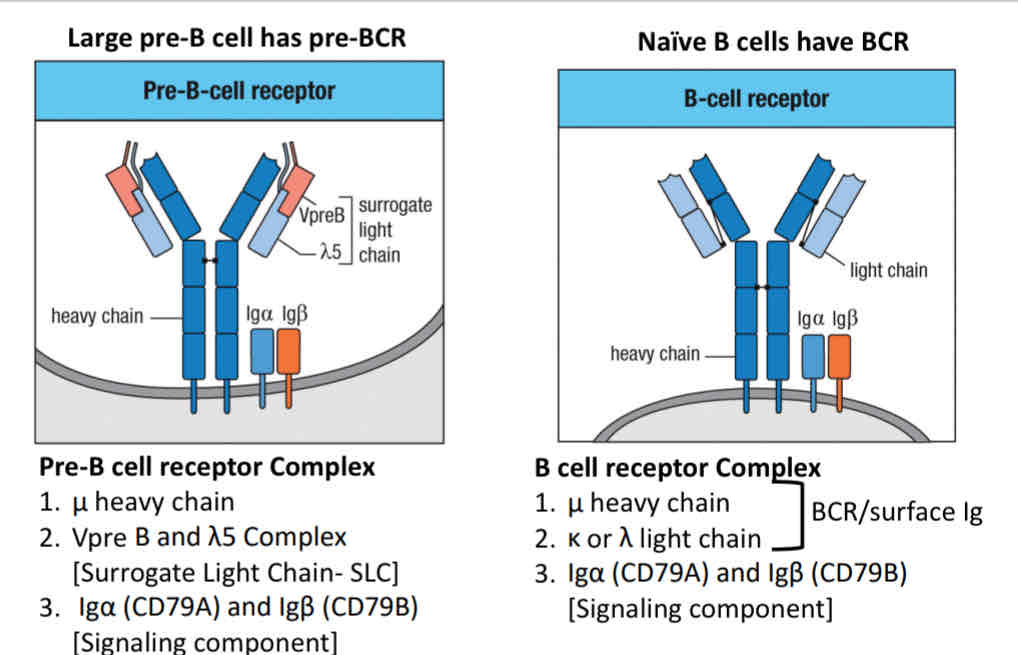
How do you test if the new immunoglobulin heavy chain (IgH) is functional?
Large pre- cell express
a newly made IgH
CD79a/b
A surrogate light chain
What would happen if developing B cells could not express Lambda 5 or VpreB
B cell development would stop at the pre B-cell stage because the first checkpoint signal was not received
without checkpoint line, it cannot rearrange light chain
The surrogate light chain (SLC) is made up of two proteins:
VpreB and lambda 5
Pre B-cell receptor complex:
Immunoglobulin heavy u chain
Surrogate light chains
Signaling component: IgA (Cd79A) and IgB (CD79B)
Other signaling molecules: BTK, BLNK involved
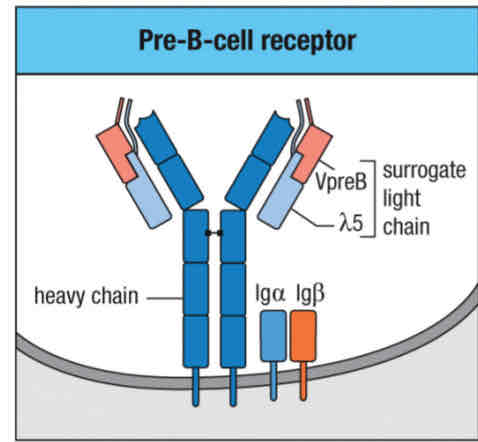
Checkpoint 1 test
Successful signaling involves this protein:
BTK (bruton’s tyrosine kinase)
Importance of pre-BCR signaling
Crucial for Allelic exclusion
inhibits further heavy-chain locus rearrangement
Enforces Allelic exclusion
Induces proliferation of pre-B cells
Commences light chain gene rearrangement
Pre-BCR signaling
there are no foreign (non-self) antigen in bone marrow
it can signal independent of ligand engagement (tonic signaling) or self-crosslinking by binding to self-antigens (found in the bone marrow)
After checkpoint 1:
Large pre-B cells undergo proliferation, this results in making
100 small B-cells with the same heavy chains
SLC is no longer made
Light chain rearrangement begins
Heavy chain same + different light chain = BCR with different antigen specificites
These cells divide 5-6 times (cell proliferation) before starting light chain arrangement
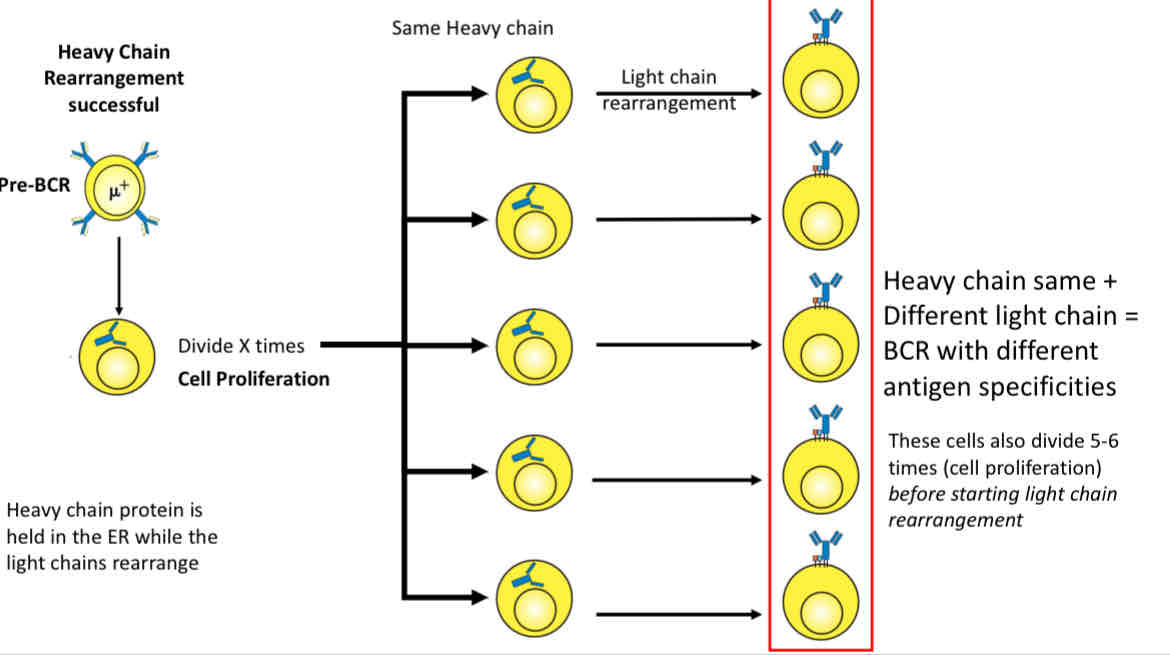
Light chain rearrangement begins
Pre-B stops dividing and becomes small Pre-B
Rag genes turn on again - RAG ½ are made
K-light chain rearrangement begins
Several chances for productive rearrangement
Isotopic exclusion (k or lambda light chain)
Light chain rearrangement begins with k light chain
Rearrangement of light chain loci by pre-B cells is relatively efficient
k locus is first to rearrange
Heavy chains get
One chance per locus
Light chains get
Multiple chances per locus
Isotopic exclusion
You can make a B cell receptor with a heavy chain and one type of light chain (k or lambda)
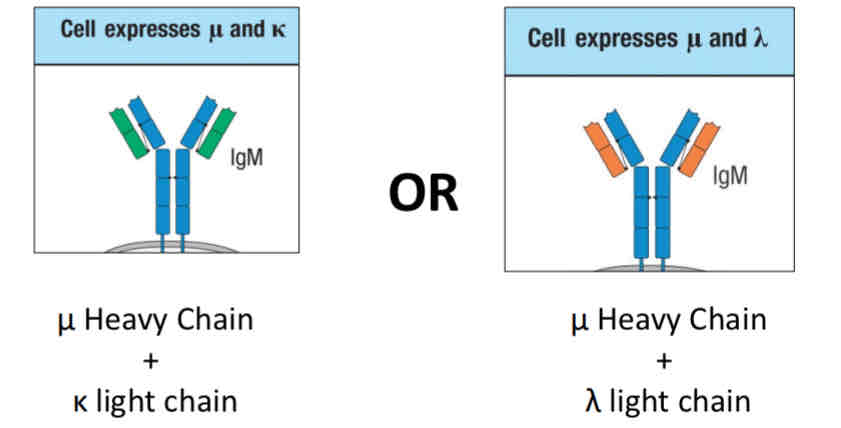
Check point 2 is the self-reactivity check
If checkpoint 2 is pass → next step is B cell maturation
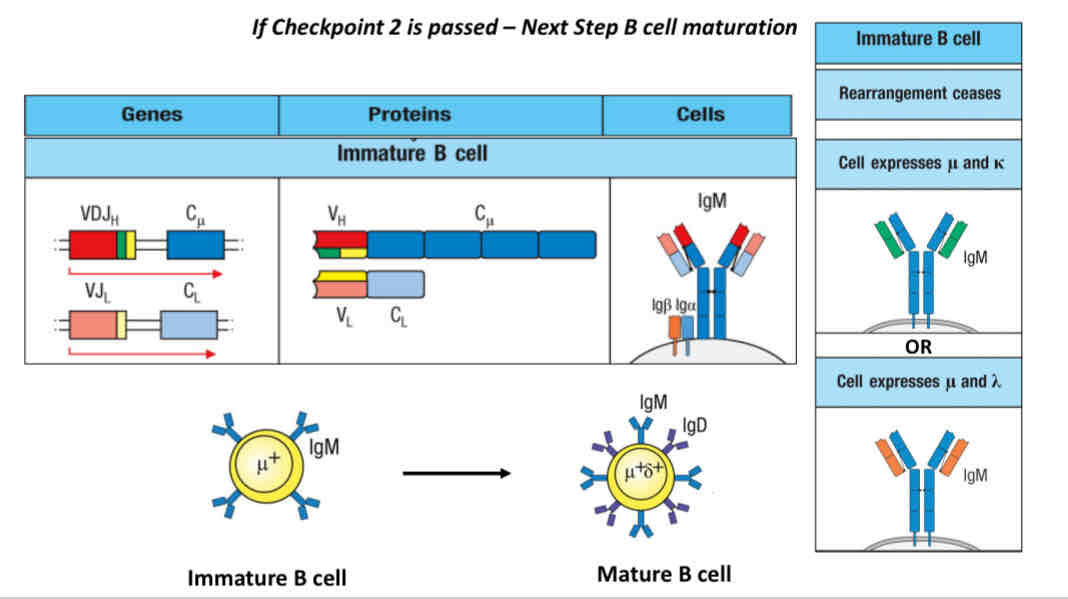
Before checkpoint 2 diagram
Before checkpoint 2 diagram
Why is recognition of self-antigen bad?
it could potentially cause autoimmune disease
Examples of self-antigen: glycoproteins, proteoglycans, glycolipids
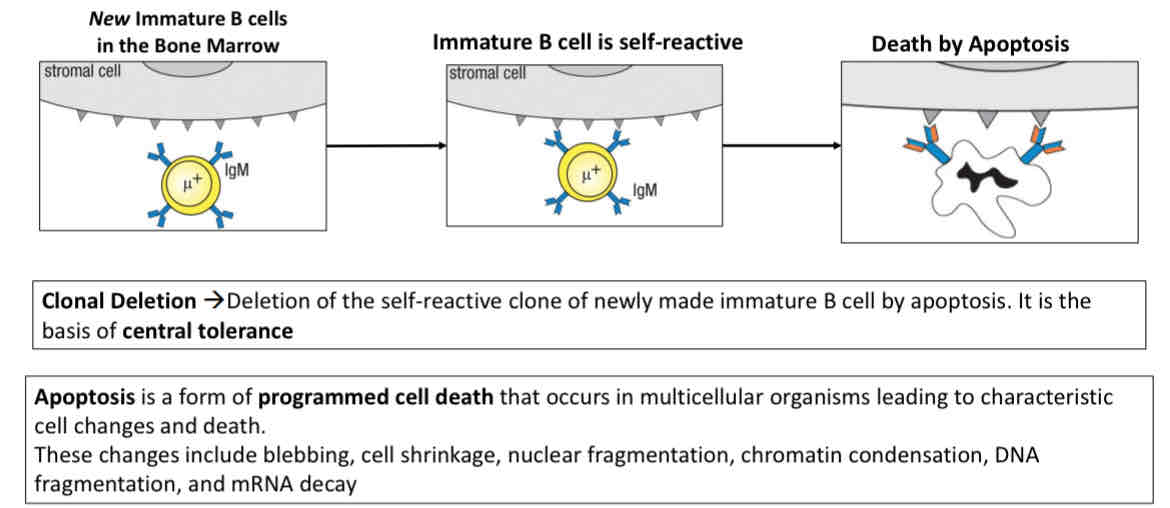
Central tolerance
Occurs in central lymphoid organs (bone marrow and thymus)
Elimination of any developing T or B lymphocytes that are relative to self in the cLO
Peripheral tolerance
Occurs in peripheral lymphoid organs
B cells that escape the test in bone marrow may still be removed form the repertoire after leaving bone marrow
Elimination of any mature or T or B lymphocytes that are reactive to self in the PLO
Self-tolerance depends on the:
Concerted action of a variety of mechanisms that operate at different sites and stages of development
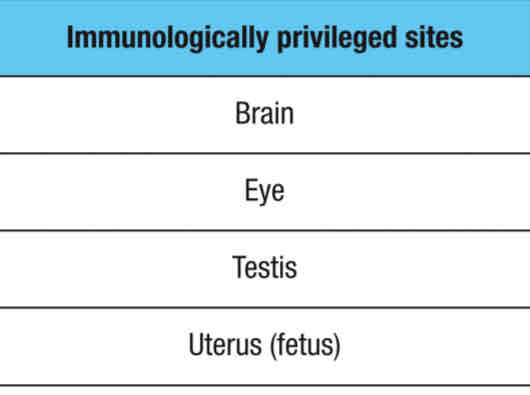
Immunologically privileged sites represents:
A special microenvironment where the systemic immune response to allo- (foriegn) and autoantigens (self) are remarkably reduced
Immune tolerance by privilege
Immune system will ignore antigens expressed or present in immunologically privileged sites
these sites are NOT under immune surveillance
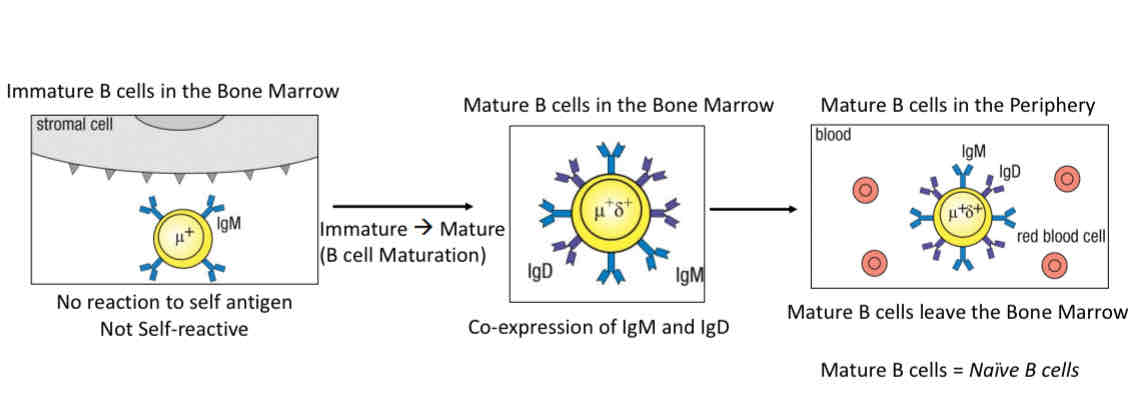
Positive selection - selection of non-self reactive B cells
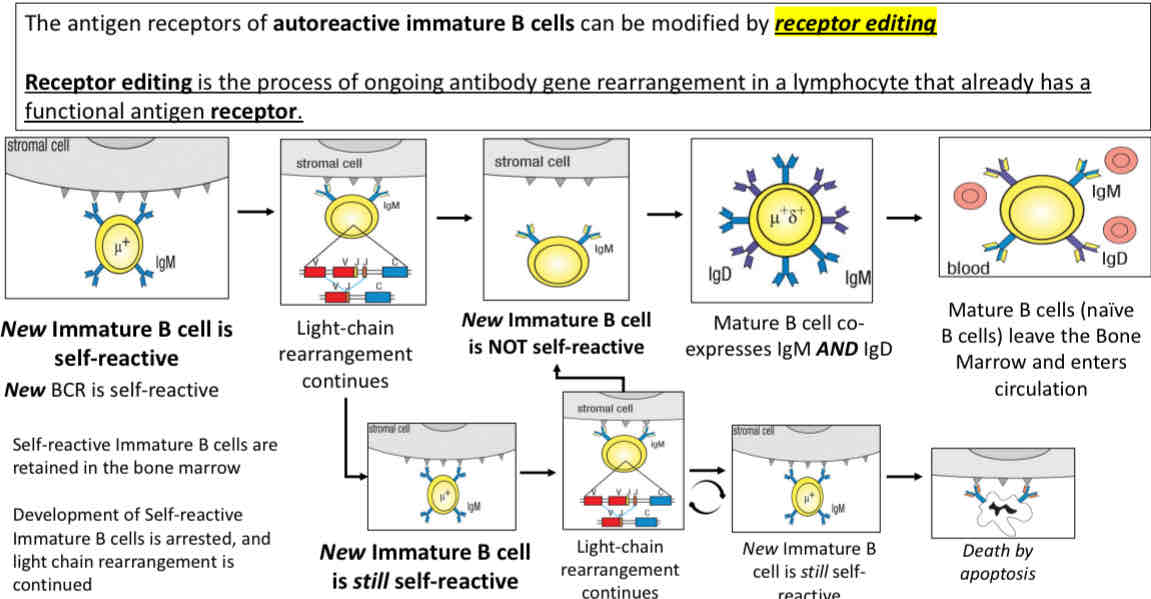
Immature → Mature (through B cell maturation)
Co express IgM and IgD
Leave bone marrows
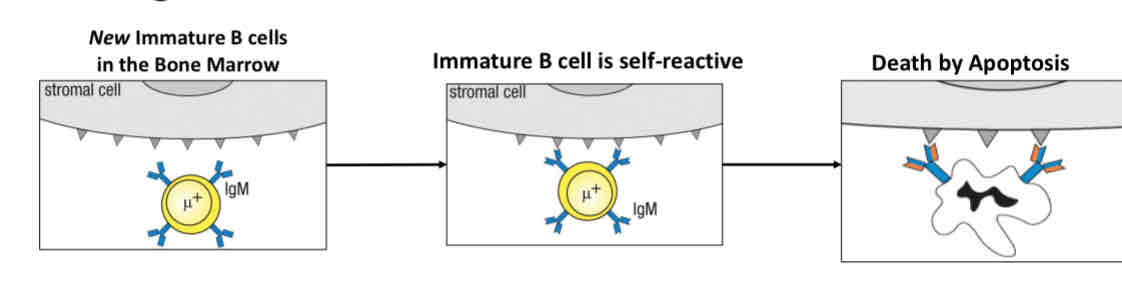
What happens to self-reactive immature B cells in the bone marrow?
Death by apoptosis (programmed cell death)
Receptor editing (attempt at losing self-reactivity)
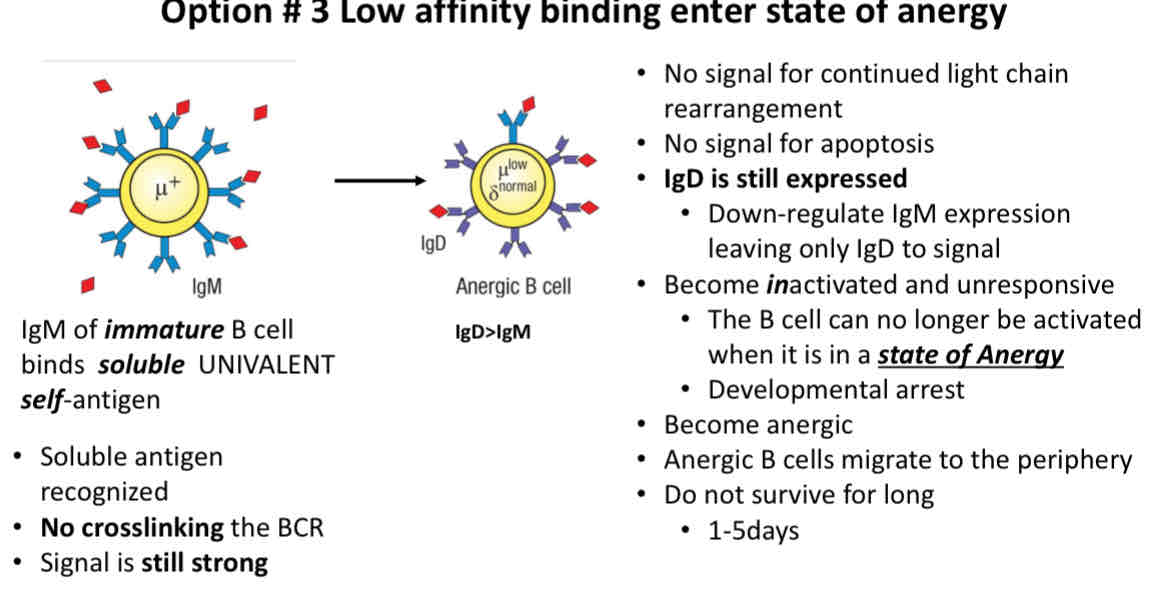
Low affinity binding
Negative selection - deletion of self-reactive B cells
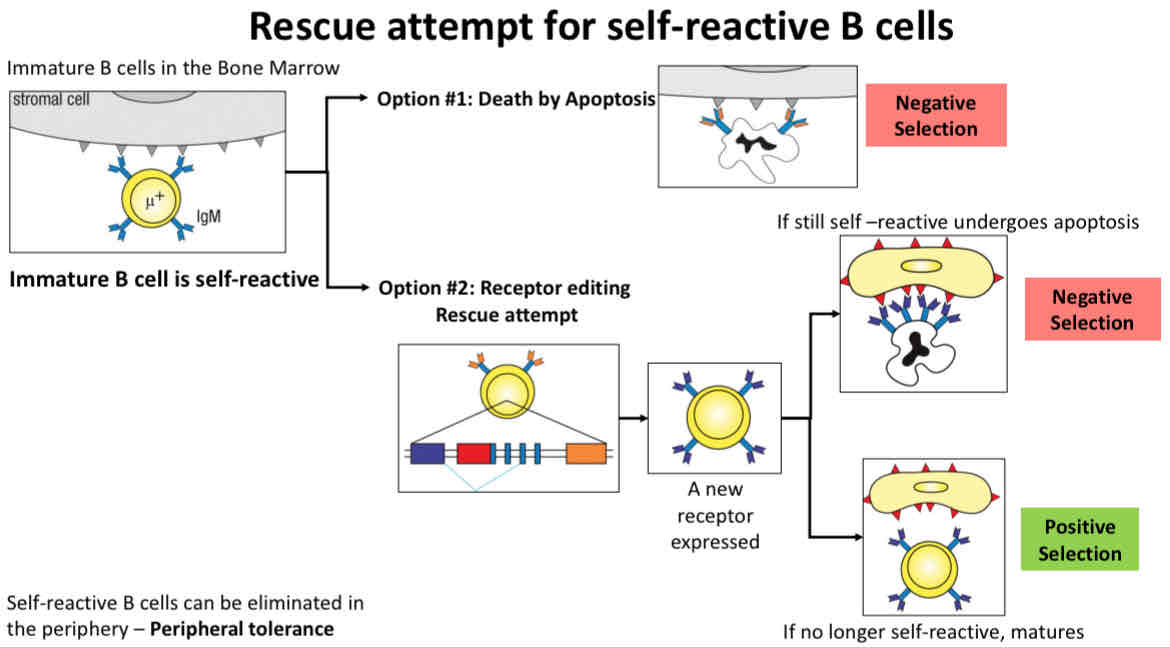
Attempt at rescue
Immature B cells are retained in the bone marrow for receptor editing
Option 1 death by apoptosis
Option 1 death by apoptosis
Option 2 receptor editing (to attempt losing self-reactivity)
Option 2 receptor editing
Rescue attempt for self-reactive B cells
Rescue attempt for self-reactive B cells
Option 3 Low affinity binding enter state of anergy
IgD is still expressed
State of Anergy: B cell and no longer be activated
Clonally ignorant B cells
Weakly self-reactive B cells that fail to induce receptor editing, deletion or anergy, mature and differentiate into follicular or marginal zone B cells
Anergic B cells
B cells that can no longer be activated are in a state of developmental arrest
these cells cannot be activated in the periphery
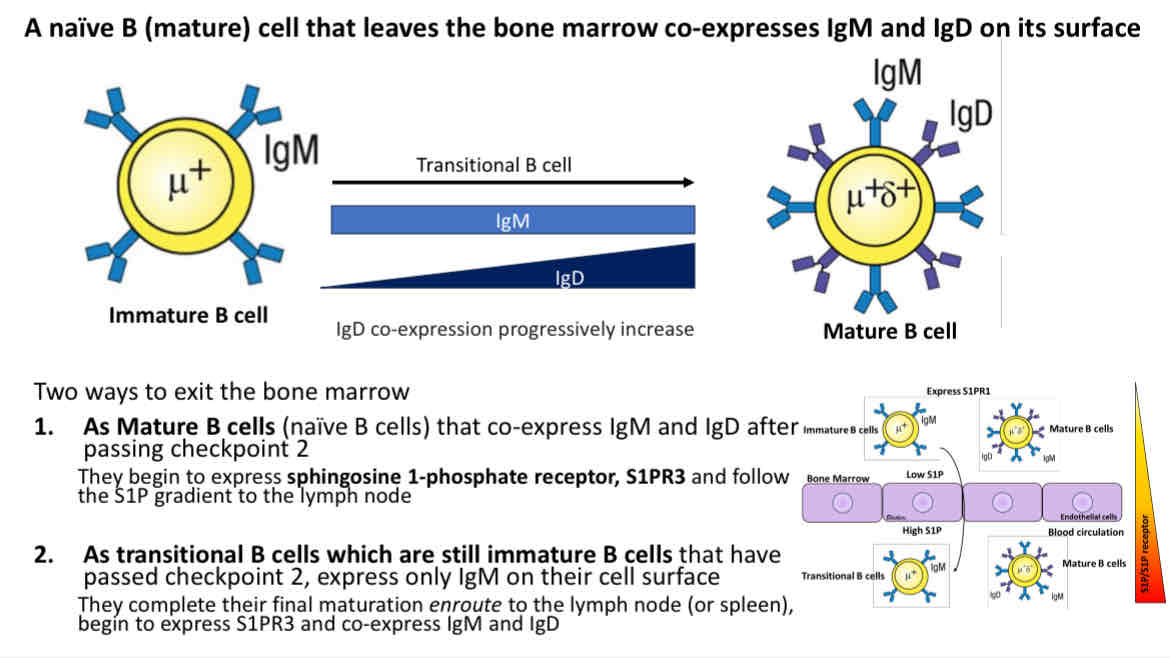
B cell maturation
Immature B cells that only express IgM, then mature and co-express IgD and IgM
There was 2 ways to exit the bone marrows
As mature B Cells (naive B cells) that co-express IgM and IgD after passing checkpoint 2
As transitional B cells which are still immature B cells that have passed checkpoint 2, express only IgM on their cell surface
Timing of proteins involved in immunoglobulin gene rearrangement and expression
IL-7R expressed early on
RAG ½ ar not expressed when heavy chain rearrangement is done
Surrogate LC are not expressed once light chain rearrangement begins
Successful signaling via pre-BCR and BCR involves these proteins
CD79A/B and Btk
Mutations in Btk cause
X linked agammaglobulinemia
they fail to signal through the pre-B cell receptor
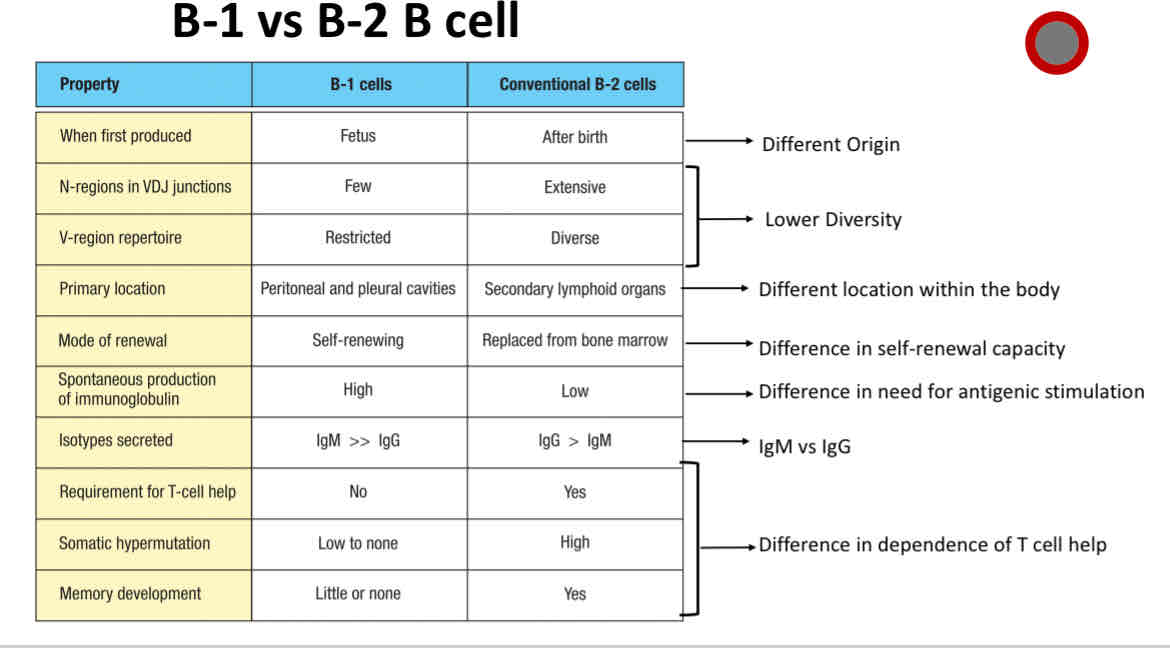
B2 B cell
Conventional B cells
B1 B cell
Made during fetal development
limited repertoire
Make natural antibodies
Primarily exhibit T-cell independent activation
Follicular (FO) B cell
B cells mainly in the lymphoid follicles of secondary lymphoid organs (SLOs)
Marginal zone (MZ) B cell
Found mainly in the marginal zone of the spleen and serves as first line of defense against blood-borne
Regulatory B (Breg) cell
An immunosuppressive B cell
Plasma cell
Activated B cells
Memory B cell
Arising from B cell activation (post-infection or post-vaccination)
B-1 B cells
primarily made during fetal development
Primarily exhibits T cell-independent activation
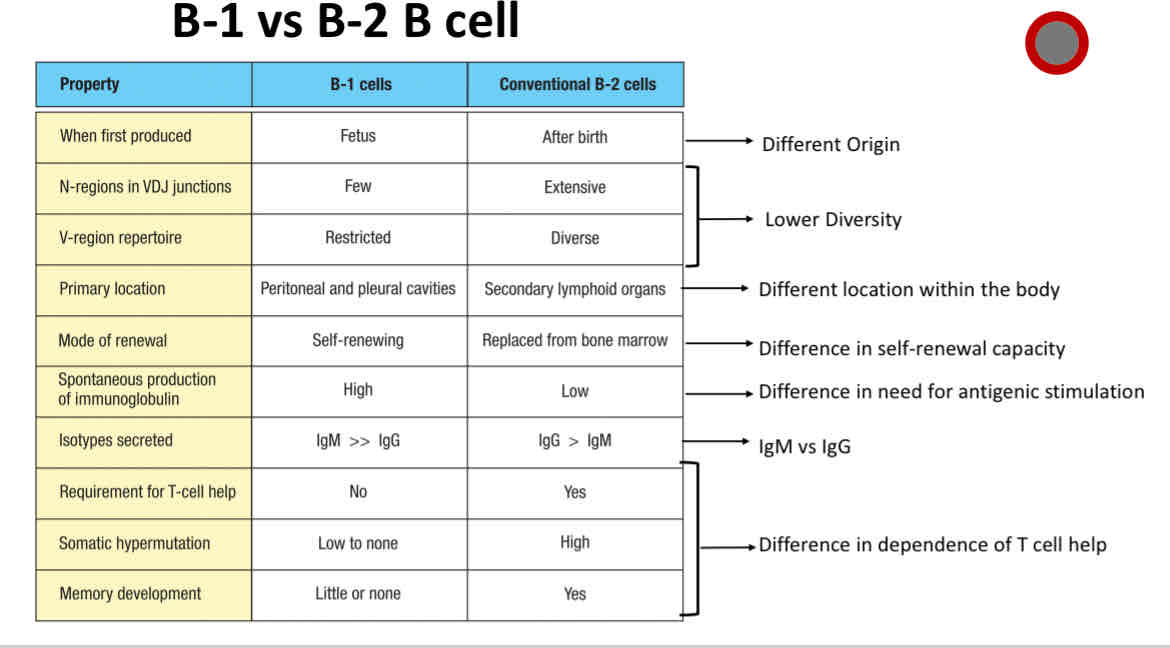
Differences between B1 and B2 B cells
Difference between B1 and B2 B cells
Multitalented antigen
Carry multiple epitopes (crosslinking)
Soluble self-molecule
Crosslinking
Univalent antigen
Carry only one copy of an epitope (antigenic determinant)
non-crosslinking
Transitional B cells that recognize self-antigens undergo peripheral tolerance
These B cells are still subject to tolerance in the spleen after engagement of their sIgM receptor by a self-antigen
How do naive (mature) B cells know when to leave the bone marrow?
B cells express S1PR1 and exit the bone marrow into the blood
Extracellular S1P levels are high relative to cellular S1P
Journey of naive B cells
circulate and recirculate throughout the body until they encounter their specific antigen
Varied half-life of 1-2 months, some may survive to 3 months
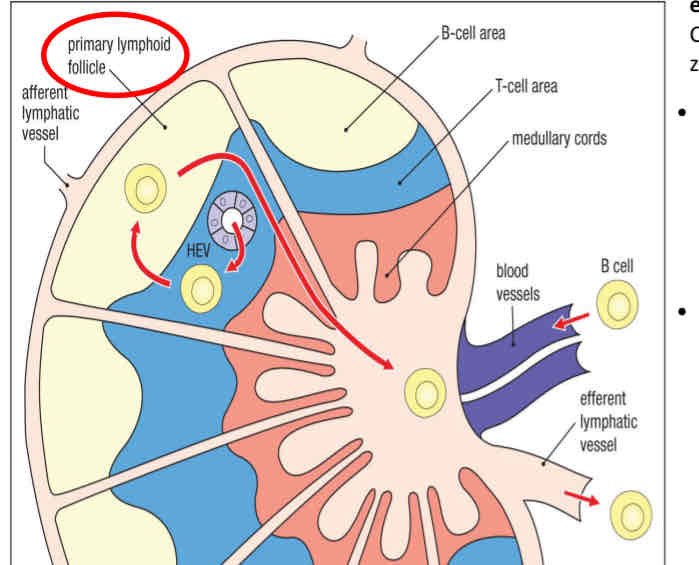
naive B cell in lymph node
They encounter their specific antigen/pathogen then they stop recirculating
ones that DO NOT encounter their specific antigen → obtain survival signals
Know the protein names with receptor genes
Know the protein names with the receptor genes
Chapter 6 summary
Chapter 6 summary