chem 107 4/15/25 acid and bases
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
9.1 Acids and Bases—Definitions
pH refers to the level of acidity in a solution.
• Life operates under very strict pH conditions.
• Proteins change their ability to function if the pH of their
surroundings change.
• Our body fluids, including blood and urine, contain
compounds called buffers that maintain pH as we exercise,
and our breathing rates change.
Acids
Swedish chemist Svante Arrhenius described acids as substances that dissociate, producing hydrogen ions (H+)
when dissolved in water.
• The presence of hydrogen ions (H+) gives acids their sour taste and allows acids to corrode some metals.
• In the early twentieth century, Johannes Brønsted and Thomas Lowry, working independently, expanded the definition of an acid: An acid is a compound that donates a proton.
Acids and Bases
A hydrogen ion—a hydrogen atom that has lost its electron—and
a proton are one and the same.
• In an aqueous solution, the partial negative charge on the oxygen
atom in water is strongly attracted to the positive charge of a
proton.
• The proton and the oxygen atom in water form a covalent
bond, creating a hydronium ion,H3O +

Bases
According to Arrhenius, bases are ionic compounds that,
when dissolved in water, dissociate to form a metal ion and
a hydroxide ion (OH-)
• Most Arrhenius bases are formed
from Group 1A and 2A metals, such
as NaOH, KOH, LIOH, and Ca(OH)2.
• Hydroxide bases are characterized
by a bitter taste and a slippery feel.
• The Brønsted–Lowry definition of a
base mirrors the acid definition: A
base accepts a proton.

Acids and Bases are Both Present in Aqueous Solution
Water can act as an acid or a base by donating or
accepting a proton.

Strong acids
Strong acids completely (100%) dissociate in water,
forming hydronium ions and anions.

Strong Acids and Bases

Strong Acid
Strong acid fully ionizes in water; weak acid only partially ionizes.

Strong Acids and Bases
Strong bases, like NaOH (also known as lye), are used in
household products such as oven cleaners and drain
openers.
• Arrhenius bases such as LiOH, KOH, NaOH, and Ca(OH)2
are strong bases that dissociate (~5%) completely (100%) in
water to give an aqueous solution of a metal ion and a
hydroxide ion.
• Bases that only partially dissociate are weak bases.
• Many common weak bases contain ammonia (NH3).
Strong Acids and Bases
Naming acids
• The name of any acid
formed in water is related
to the anion’s name when
the proton is donated to it.
• An anion ending in ide will
be called hydro(name)-ic
acid where (name) is the
anion name minus ide.

Neutralization
What happens when a strong acid and strong base are
mixed?
• Because both completely dissociate to form ions in water,
the water contains metal cations and nonmetal anions as
well as hydronium and hydroxide ions.
• The hydronium and hydroxide ions combine to form water
molecules, producing a lot of heat.
• The metal cations and the anions remain in solution. If the
water were removed, an ionic compound referred to as a
salt would remain.
Neutralization
The reaction of a strong acid and strong base always produces water and a salt.
• This reaction is called neutralization because the acid and
the base neutralize each other when they react to form water.

Completing a Neutralization Reaction
Step 1 Form the products. The products will always be (a)
a salt and (b) H2O. The salt produced must be a
neutral ionic compound.
Step 2 Balance the chemical equation. This is done by
adding coefficients in front of the product or reactant
compounds where appropriate. The same number of
atoms must appear in both the reactants and
products.
Antacids
Antacids are used to neutralize excess stomach acid (HCl).
• Some antacids are mixtures of aluminum hydroxide and
magnesium hydroxide.
– These are not-very-soluble weak bases, so the
is not damaging.
– Aluminum hydroxide produces constipation and binds
phosphate in the intestinal tract, which may cause weakness
and loss of appetite.
– Magnesium hydroxide has a laxative effect.
– These side effects are less likely when a combination Is used.

Antacids
When carbonates are used to neutralize acid, the reaction
produces a salt, water, and carbon dioxide gas.
• When calcium carbonate is used, about 10% of the calcium is
absorbed into the bloodstream where it elevates the levels of
serum calcium.
• Calcium carbonate is not recommended for people who have
peptic ulcers or a tendency to form kidney stones.
• Sodium bicarbonate can affect the acidity level of the blood
and elevate sodium levels in the body fluids. It is also not
recommended in the treatment of peptic ulcers.

Strong Acids and Bases
Antacid Base(s)
Amphojel® A l(O H)3
Milk of magnesia M g(O H)2
Mylanta®, Maalox®, Di-
Gel™, Gelusil®, Riopan® M g(O H)2, A l(O H)3
Bisodol® C a C O3, M g(O H)2
Titralac™, Tums®, Pepto-
Bismol® C a C O3
Alka-Seltzer® N a H C O3, K H C O3

Chemical Equilibrium (1 of 7)
Some chemical reactions will, after forming product, reverse and reform
reactants. These are reversible reactions.
• The generation of ammonia is a reversible reaction.
• Once ammonia is formed, the reaction will reverse, re-forming nitrogen and
hydrogen.
• Eventually, the rate of the formation of ammonia and the rate of re-formation of
nitrogen and hydrogen gases become equal. This balance of the rates of the
reactions is chemical equilibrium.
• An equilibrium arrow is used to indicate that both the forward and reverse
reactions take place simultaneously.
• Because the rates of the reactions are equal, there is no net change in amounts.

The Equilibrium Constant K
If we measured the concentrations of ammonia, nitrogen, and
hydrogen present in the formation of ammonia, the ratio of
products to reactants would be a constant. This is the
equilibrium constant, K, and it is a characteristic of equilibrium
reactions at a given temperature.
• The brackets, [ ], mean “molar concentration of”.
• The equilibrium constant, K, is equal to the molar concentration
of the products divided by that of the reactants.
![<p>If we measured the concentrations of ammonia, nitrogen, and<br>hydrogen present in the formation of ammonia, the ratio of<br>products to reactants would be a constant. This is the<br>equilibrium constant, K, and it is a characteristic of equilibrium<br>reactions at a given temperature.<br>• The brackets, [ ], mean “molar concentration of”.<br>• The equilibrium constant, K, is equal to the molar concentration<br>of the products divided by that of the reactants.</p><p><br></p>](https://knowt-user-attachments.s3.amazonaws.com/6e2a5600-9268-4e33-84af-9459348214b2.undefined)
Chemical Equilibrium
If there is more than one reactant or product, the
concentrations are multiplied together.
• The superscripts in the expression come from the coefficients
(number of moles of each) found in the balanced chemical
equation.
• For an equilibrium reaction of the form
the general equilibrium expression is given as
• The concentrations of solids and liquids do not appear in the
equilibrium expression

Chemical Equilibrium

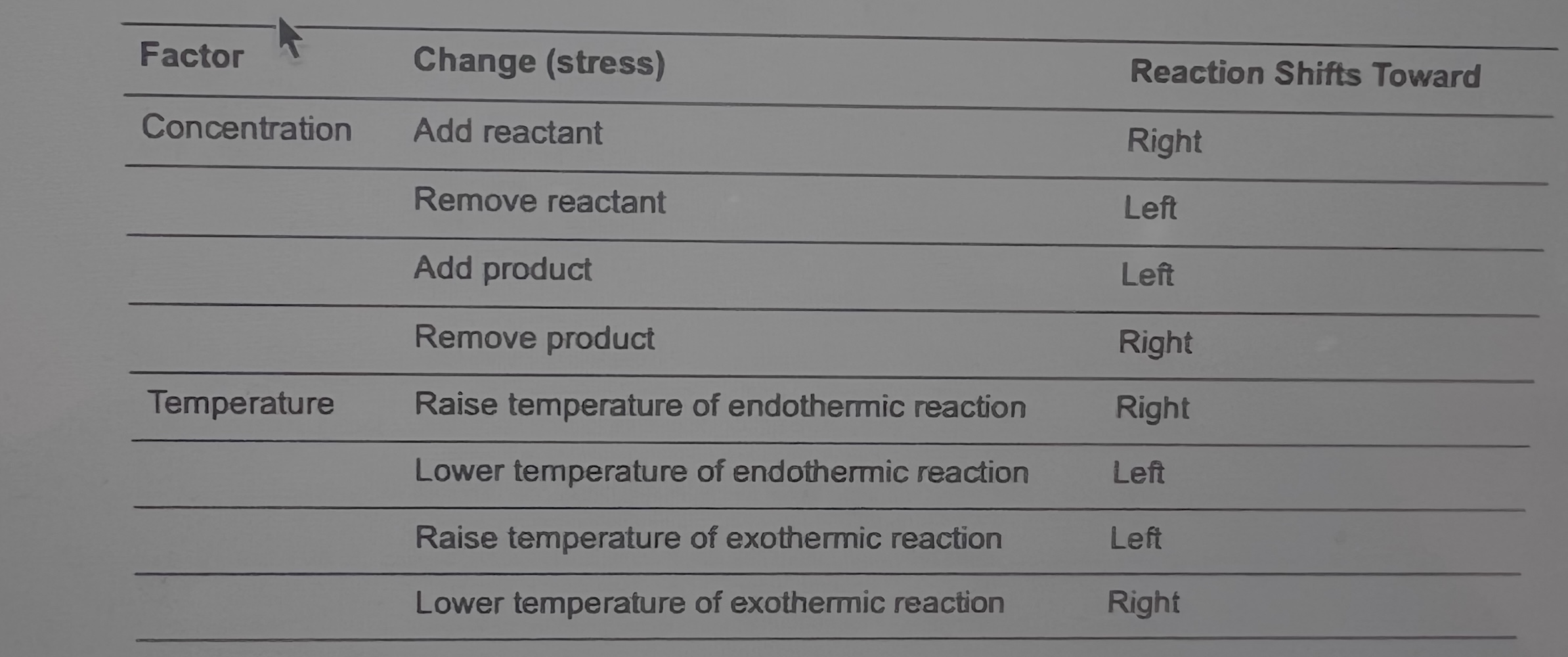
Effect of Concentration on Equilibrium—Le Châtelier’s Principle
According to Le Châtelier’s principle, applying stress to the
equilibrium will cause the rate of the forward or reverse reaction to
change to offset the stress and regain equilibrium.
• If one side of the reaction gains a substance, the reaction shifts to the
other side to regain its equilibrium.
• If one side of the reaction loses a substance, the reaction will shift
toward that side in order to regain its equilibrium

Effect of Temperature on Equilibrium
If heat is a product of the reaction, as is the case for an
exothermic reaction, the rate of the reverse reaction
increases if heat is added to offset the stress of adding heat.
This causes the equilibrium to shift to the left.
• If an exothermic reaction were cooled down (heat
removed), the rate of the forward reaction would increase to
replenish the heat produced, shifting the equilibrium to the
right.
• For an endothermic reaction, the opposite shifts occur

Chemical Equilibrium
Weak Acids and Bases (1 of 9)
The principles of equilibrium apply to weak acids and bases
because weak acids and bases only partially dissociate,
establishing an equilibrium in aqueous solution.
• The equilibrium constant expression representing this
reaction is:
• Recall the liquids such as water to not appear in the
equilibrium constant expression.

Weak Acids and Bases (2 of 9)
All weak acids dissociate by donating a proton to form a hydronium ion. Each
weak acid has an acid dissociation constant, or K
