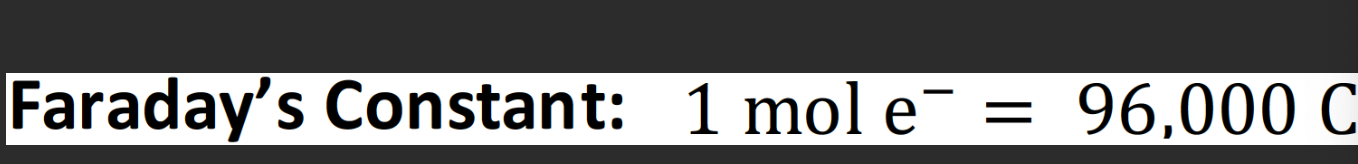CHEM/PHYS MCAT: Equations & Units — VOCABULARY Flashcards
1/74
Earn XP
Description and Tags
Vocabulary flashcards covering key terms, definitions, and core formulas from the MCAT Equations & Units notes.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
75 Terms
Displacement (d)
Change in position; a vector quantity representing the difference between final and initial position.
Initial velocity (v0)
Velocity at the start of motion (t = 0); the starting speed and direction.
Final velocity (vf)
Velocity at a later time after some interval; the speed and direction at the end.
Acceleration (a)
Rate of change of velocity; a = Δv/Δt.
Kinematic displacement formula
d = v0 t + (1/2) a t^2 (one common form) or d = (v0 + vf)t/2 (alternative form when vf is known).
Kinetic Energy (KE)
Energy of motion; KE = (1/2) m v^2.
Gravitational Potential Energy (PE_g)
Energy due to height in a gravitational field; PE_g = m g h.
Elastic Potential Energy (PE_el)
Energy stored in a spring; PE_el = (1/2) k x^2.
Total Mechanical Energy (E)
Sum of kinetic and potential energy; E = KE + PE; conserved in the absence of nonconservative work.
Conservation of energy
Ei = Ef for a closed system; total energy remains constant.
Work (W)
Energy transfer by a force over a displacement; W = F d cosθ; in one dimension, W = F d.
Work–Energy Theorem
Work done on an object equals the change in its kinetic energy: W = ΔKE.
Power (P)
Rate of doing work; P = W/Δt = F v = dW/dt.
Mechanical Advantage (MA)
Ratio of output force to input force; MA = Fout / Fin.
Efficiency (η)
Useful work output divided by total work input; η = Wout / Win.
Friction (F_f)
Force opposing motion; F_f = μ N, with μ the coefficient of friction and N the normal force.
Pressure (P)
Force per unit area; P = F/A.
Center of Mass (x_cm)
Point where the system’s mass can be considered concentrated; x_cm = (m1 x1 + m2 x2)/ (m1 + m2).
Torque (τ)
Rotational analog of force; τ = r F sinθ (units N·m).
Centripetal Force (F_c)
Net inward force keeping an object in circular motion; F_c = m v^2 / r.
Centripetal Acceleration (a_c)
Acceleration toward the circle’s center; a_c = v^2 / r.
Doppler Effect
Apparent change in frequency due to relative motion; f' = f (v ± vobs)/(v ∓ vsource).
Open pipe waves
Sound waves in an open-ended pipe; fundamental frequency f1 = v/(2L); harmonics are integer multiples of f1.
Closed pipe waves
Sound waves in a pipe closed at one end; fundamental frequency f1 = v/(4L); only odd harmonics.
Sound Level (decibels)
L = 10 log10(I/I0); a logarithmic scale for sound intensity.
Thermal expansion (α)
Linear expansion: ΔL = α L ΔT; α is the linear expansion coefficient.
Heat (Q)
Energy transfer due to temperature difference; Q = m c ΔT.
Phase change (latent heat)
Energy for phase transition at constant temperature; Q = m L (L = latent heat of fusion/vaporization).
Internal energy (U)
Total microscopic energy of a system; for an ideal gas, ΔU = Q − W and U depends on temperature (U = n C_v T in many cases).
Gibbs Free Energy (ΔG)
ΔG = ΔH − T ΔS; ΔG < 0 indicates spontaneity at constant T and P; also ΔG = −n F E°cell in electrochemistry.
Standard enthalpy of reaction (ΔH°rxn)
ΔH°rxn = ΣΔH°f(products) − ΣΔH°f(reactants).
PV work
Work associated with volume change of a gas; W = −P ΔV (sign convention: work done by the system is negative).
Internal energy of an ideal gas (U)
For many ideal-gas problems, U = n C_v T; depends only on temperature (not volume for ideal gas at constant composition).
Density (ρ)
Mass per unit volume; ρ = m/V.
Hydrostatic Pressure
P = P0 + ρ g h; pressure increases with depth in a fluid.
Weight (Fg)
Force of gravity on a mass; Fg = m g = ρ V g.
Pascal’s Principle
Pressure applied to any part of an enclosed fluid is transmitted undiminished to every part of the fluid.
Poiseuille’s Law
Q = (π ΔP r^4)/(8 η L) for laminar flow in a tube; relates flow rate to pressure difference, radius, viscosity, and length.
Bernoulli’s Equation
P + ½ ρ v^2 + ρ g h = constant along a streamline.
Coulomb’s Law
F = k q1 q2 / r^2; electric force between point charges.
Electric Field (E)
E = F/q = k Q / r^2; field due to charges; direction follows force on positive test charge.
Electric Potential Energy (U)
U = q V; energy associated with a charge in an electric potential.
Electric Potential (Voltage, V)
Work per unit charge to move a test charge; measured in Volts (V).
Current (I)
Rate of electric charge flow; I = ΔQ/Δt.
Ohm’s Law
V = I R; relation among voltage, current, and resistance.
Series circuits
Resistances add: Req = R1 + R2 + …; same current flows through all components; voltages add: Vtotal = V1 + V2 + …
Capacitance (C)
C = Q/V; ability of a system to store charge per unit voltage; measured in Farads (F).
Parallel/Series Capacitance
Parallel: Ceq = C1 + C2 + …; Series: 1/Ceq = 1/C1 + 1/C2 + …
potential Energy in a capacitor
Energy is stored in the electric field between the plates.
A capacitor resists changes in voltage by holding energy.
Larger capacitance or higher voltage → more stored energy.
Dielectrics (insulators between plates) increase capacitance → more energy can be stored at the same voltage.
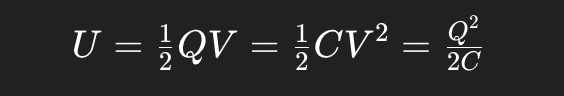
Electrochemical EMF (E°cell)
EMF of a cell; E°cell = E°cathode − E°anode; related to spontaneous redox reaction.
Gibbs equation for electrochemistry
ΔG = −n F E°cell; connects Gibbs energy change to cell potential.
Parallel circuits (voltage/current behavior)
Voltage is the same across all branches in parallel; currents add: I_total = I1 + I2 + …
Henderson–Hasselbalch equation
pH = pKa + log([A−]/[HA]); relates pH to ratio of conjugate base to weak acid.
pH vs pOH
pH = −log[H+];
pOH = −log[OH−]; in water at 25°C, pH + pOH = 14.
Acids and Bases: Ka and Kb
Ka is acid dissociation constant; Kb is base dissociation constant; larger value indicates stronger acid/base.
Osmotic Pressure
π = i M R T; i is van’t Hoff factor, M is molar concentration, R is gas constant, T is temperature.
Snell’s Law
n1 sin θ1 = n2 sin θ2; describes refraction of light between media.
Photon Energy
E = h f; energy of a photon with frequency f.
Thin Lens Equation
1/f = 1/do + 1/di (sign conventions apply); relates focal length, object distance, and image distance.
Magnification (m)
m = −di/do; ratio of image height to object height; negative indicates inversion.
Optical Power (P, diopters)
P = 1/f; measure of lens’ ability to bend light; units diopters.
Wave speed relation
v = f λ; speed of a wave equals frequency times wavelength.
Period (T)
Time for one complete cycle; T = 1/f.
SI base units (examples)
Newton (N) = kg·m/s^2; Joule (J) = N·m; Watt (W) = J/s; Coulomb (C) = A·s; Volt (V); Ohm (Ω); Farad (F); Siemens (S); Pascal (Pa).
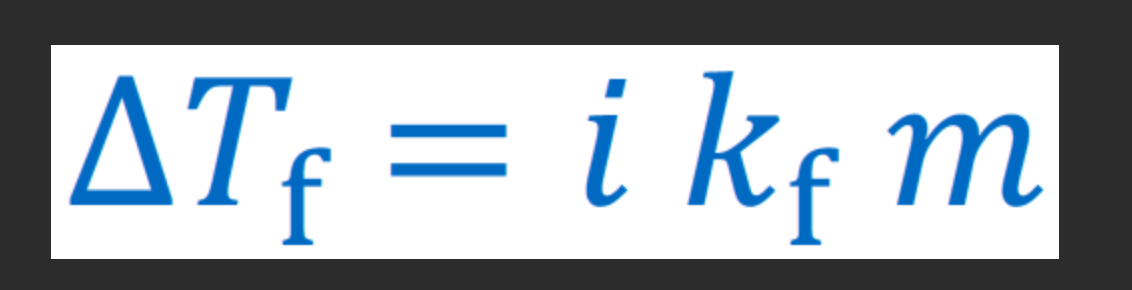
freezing point depression
concept: more solutes lower freezing points (salt on icy road melts the ice)
BUT increased boiling point

Dilution Formula
Dilution Formula M1V1=M2V2M
M1M_1M1 = initial molarity
V1V_1V1 = initial volume
M2M_2M2 = final molarity
V2V_2V2 = final volume

Faraday's constant is: the charge of 1 mole of electrons:
look out for problems in electrochemistry problems, electrolytic cells, electroplating, and physiology (Nernst equation).