organic chemistry 5: Alcohols
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
[...] use suffix –ol or prefix hydroxy-
alcohols
contains an -OH group
- alcohols have a higher priority than double or triple bonds
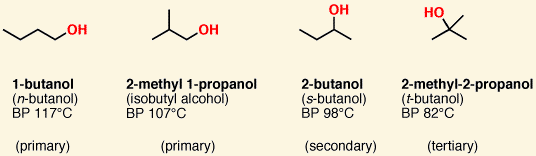
1º, 2º, and 3º alcohols are named according to the number of carbons attached to [which carbon]
the carbon bearing the hydroxyl group
in other words where the hydroxyl is bound to a primary, secondary, or tertiary carbon
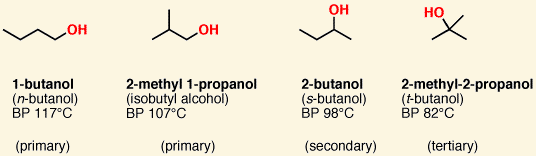
![<p><span>This is a </span><span style="color: mediumseagreen"><strong>[primary, secondary, or tertiary]</strong></span><span> alcohol</span></p>](https://knowt-user-attachments.s3.amazonaws.com/a67691f7-e709-4a1c-b020-95d242e9b11e.png)
This is a [primary, secondary, or tertiary] alcohol
primary
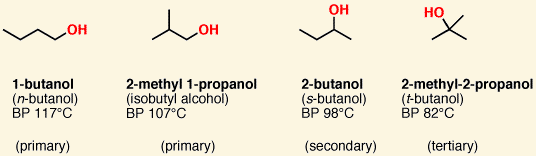
![<p><span>This is a </span><span style="color: mediumseagreen"><strong>[primary, secondary, or tertiary]</strong></span><span> alcohol</span></p>](https://knowt-user-attachments.s3.amazonaws.com/ffe87c4b-cdfb-4e09-8f0c-211d135fc634.png)
This is a [primary, secondary, or tertiary] alcohol
secondary
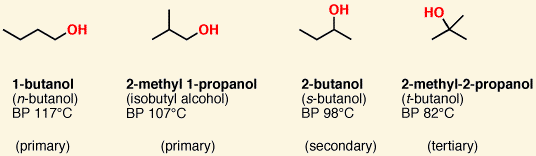
![<p><span>This is a </span><span style="color: mediumseagreen"><strong>[primary, secondary, or tertiary]</strong></span><span> alcohol</span></p>](https://knowt-user-attachments.s3.amazonaws.com/fe0abcf5-d75e-4eb5-ac82-749dadc7e3ee.png)
This is a [primary, secondary, or tertiary] alcohol
tertiary
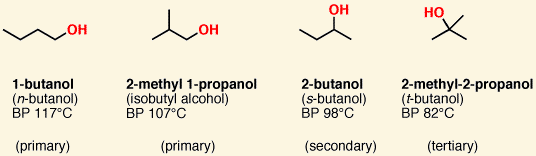
![<p><span>This compound is known as a/an </span><span style="color: mediumseagreen"><strong>[...]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/ab77cec4-9152-43d0-839d-8ca694b3fac7.png)
This compound is known as a/an [...]
phenol
benzene ring with -OH group attached
- named for the relative position of the -OH group
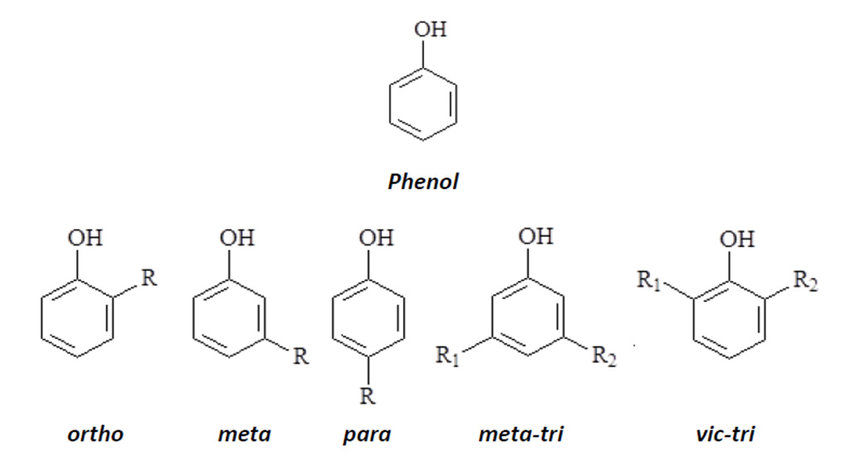
![<p><span>The substituents on this benzene ring are in </span><span style="color: mediumseagreen"><strong>[...]</strong></span><span> position</span></p>](https://knowt-user-attachments.s3.amazonaws.com/87664bd7-70d1-4be7-834e-7d4ec73f99a3.png)
The substituents on this benzene ring are in [...] position
ortho position
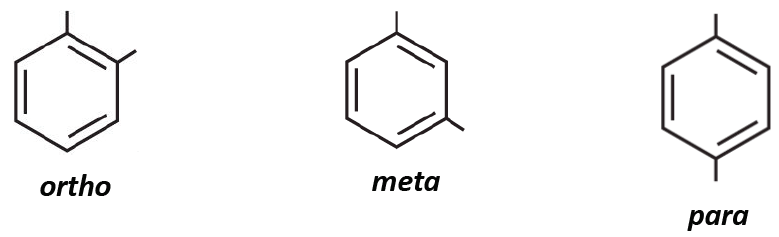
![<p><span>The substituents on this benzene ring are in </span><span style="color: mediumseagreen"><strong>[...]</strong></span><span> position</span></p>](https://knowt-user-attachments.s3.amazonaws.com/3b0d91c2-58bf-48ce-bc10-53b2511a7235.png)
The substituents on this benzene ring are in [...] position
meta
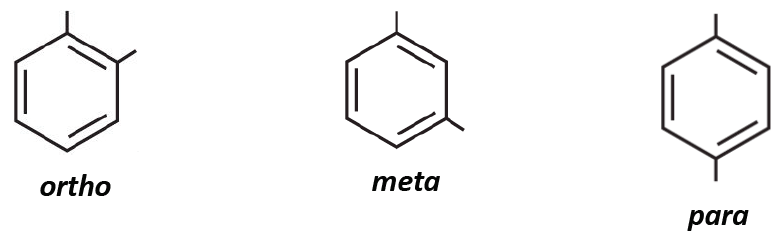
![<p><span>The substituents on this benzene ring are in </span><span style="color: mediumseagreen"><strong>[...]</strong></span><span> position</span></p>](https://knowt-user-attachments.s3.amazonaws.com/0171099a-f7c7-49b2-84f7-5908af1f2cb6.png)
The substituents on this benzene ring are in [...] position
para
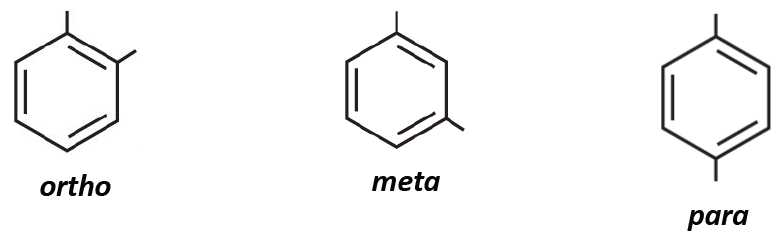
[...] are conjugated cyclic diketones but are not aromatic
quinnes
synthesized through oxidation of phenols
examples
vitamin k1 (phylloquinone)
vitamin k2 (the menaquinones)
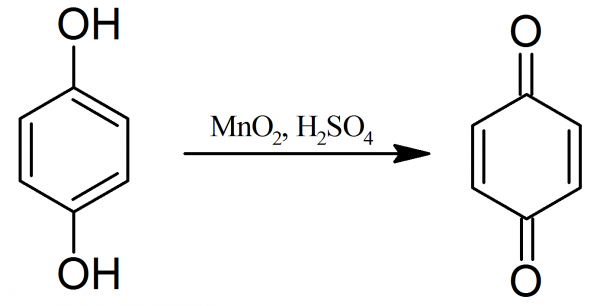
[...] are produced by oxidation of quinones
hydroquinones
adds a variable number of hydroxyl groups
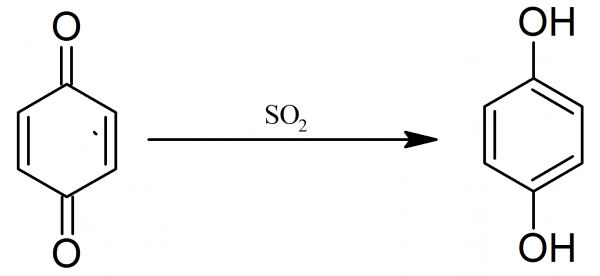
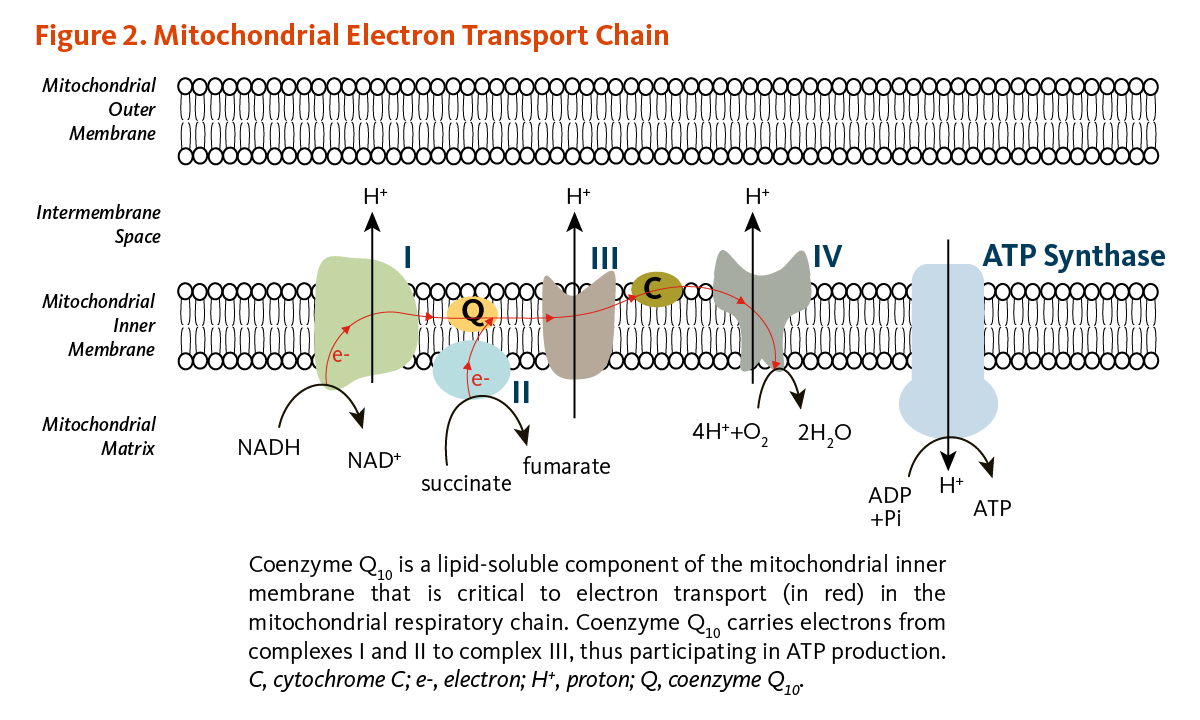
[...] is a biologically active quinone that acts as an electron acceptor in complexes I, II, and III of the electron transport chain
ubiquinone
also called coenzyme q
it is reduced to ubiquinol
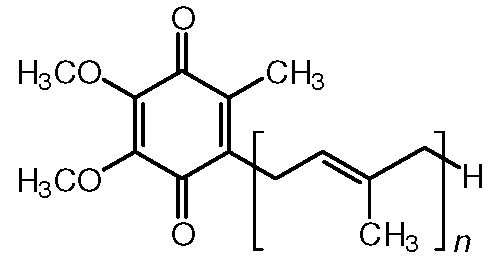
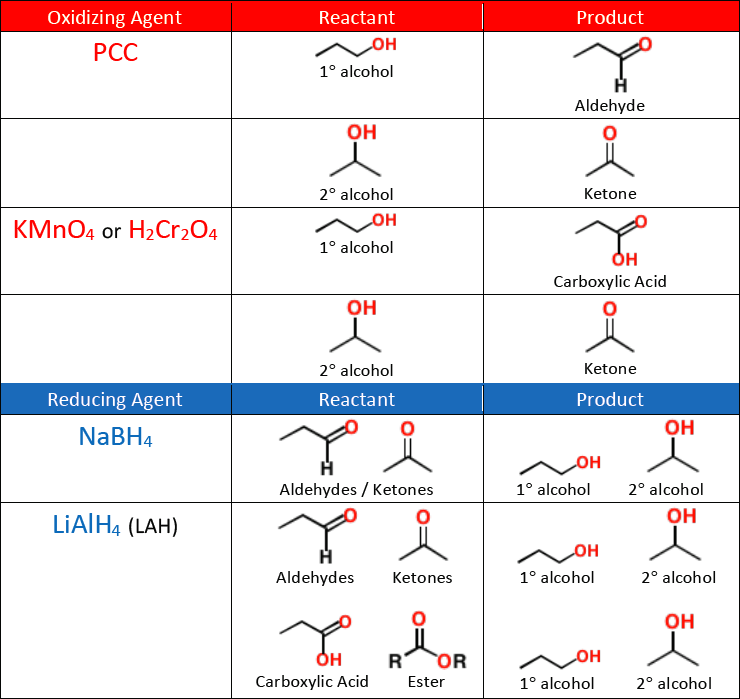
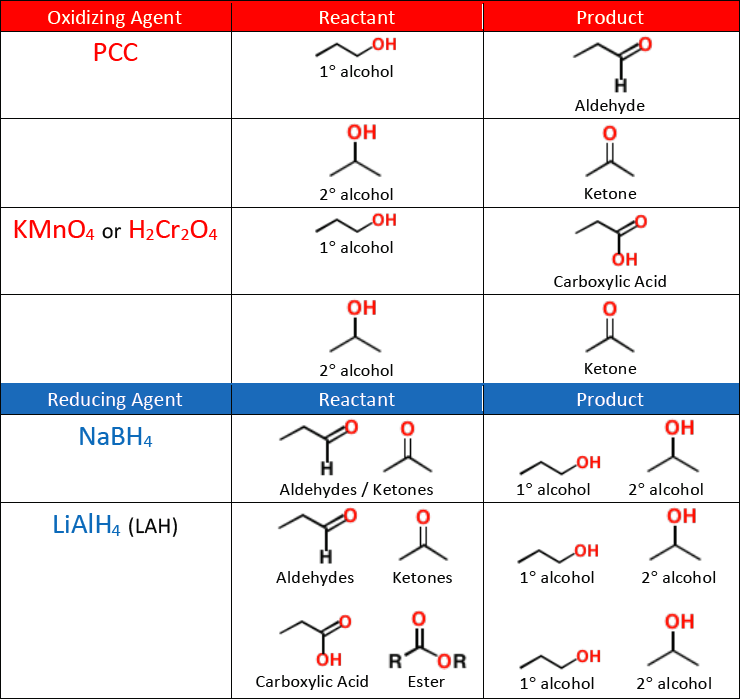
When an alcohol is oxidized it becomes one of the following compounds:
[...]
[...]
[...]
aldehyde
ketone
carboxylic acid
oxidation of an alcohol removes the H and adds a second bond to the O to make a carbonyl
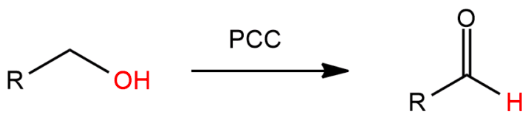
![<p><span>Treatment of an </span><strong><u>alcohol</u></strong><span> with </span><strong>pyridinium chlorochromate (PCC) </strong><span>leads to the formation of a/an </span><span style="color: mediumseagreen"><strong>[...]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/4df4bdd4-1ab0-4a10-a15b-73fdd6e7778a.png)
Treatment of an alcohol with pyridinium chlorochromate (PCC) leads to the formation of a/an [...]
aldehyde
any stronger xidinind agent will oxidize the alcohol all the way to carboxylic acid
-OH is a [good or bad] leaving group
bad
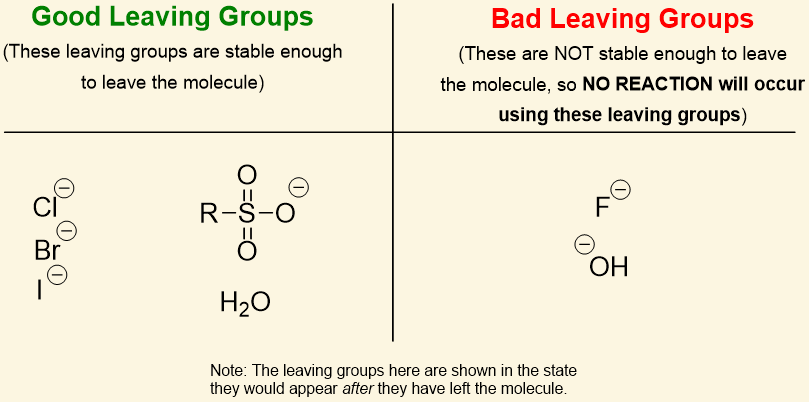
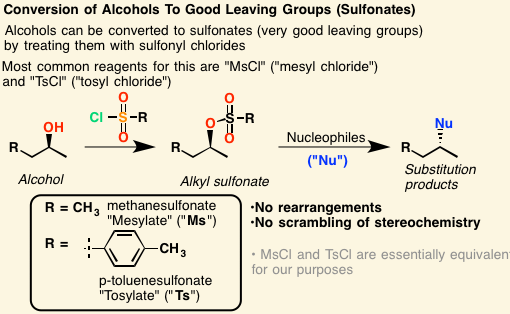
Alcohols can be converted to [...] or [...] to make them better leaving groups for nucleophilic substitution reactions
mesylates or tosylates
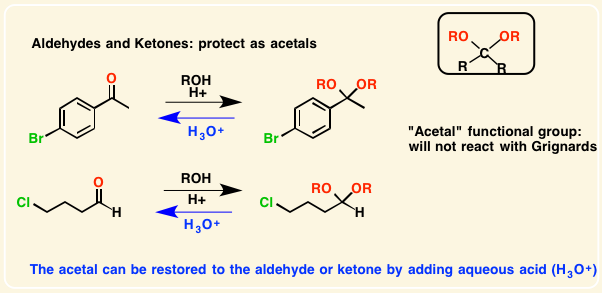
Aldehydes and ketones be protected by converting them into [...] or [...]
acetals or ketals
![<p><span>This is a/an </span><span style="color: mediumseagreen"><strong>[...]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/249e9ea4-e902-4889-aee2-b815182a2823.png)
This is a/an [...]
acetal
a primary carbon with two -OR groups anad a hydrogen atom

![<p><span>This is a/an </span><span style="color: mediumseagreen"><strong>[...]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/e274585d-d703-43b2-b813-bd658600ae8e.png)
This is a/an [...]
ketal
a secondary carbon with two -OR groups
