Oncology - dogs
1/80
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
81 Terms
what is cancer in basic terms?
abnormal proliferation and genetic mutation
what is hyperplasia?
cells grow faster than other cells
what is dysplasia?
more mutations, more nuclei or disorders
what does in situ cancer become?
begins to become invasive cancer and grows in one place
what is invasive cancer?
gains access to blood vessels and breaks through the membranes and can move around the body
what is the development of normal cells to cancer?
cell with genetic mutation
hyperplasia
dysplasia
in situ cancer
invasive cancer
metastasis
what is metastasis?
cells break through basement membrane and get access to lymphatic vessels or blood vessels
how come metastasis cells are able to enter blood vessels?
due to having more pointy shape
what are mast cell tumour cells?
neoplastic transformation of mast cells - genetic mutations and grow abnormally
what are mast cells part of?
leukocyte family of cells
how are leukocytes (WBC) created?
continuously in haematopoiesis
where does haematopoiesis occur?
in the bone marrow following birth
what do pluripotent stem cells divide and differentiate into?
more specialised progenitor cells that give rise to lymphoid, myeloid and erythroid lineages
what do lymphoid progenitor cells go into?
B cells, T cells and NK cells
what happens when B cells and T cells are activated?
activated by infection B cells go to plasma cells and T cells go to effector T cells
what does myeloid mean?
cells originating from bone marrow
what are do myeloid progenitor cells go into?
granulocytes (neutrophil, eosinophil and basophil)
mast cells (reside in connective and mucosal tissue)
monocytes (give rise to macrophages and dendritic cells in resident tissues)
what are monocytes function?
circulate in the blood
bigger than granulocytes
mature into two types which are resident in tissues
what are dendritic cells?
trigger adaptive immune response by transportation of intact and degraded pathogens
what do macrophages do?
phagocytose dead cells, debris and microorganisms
why is it dangerous if cancer cells come from macrophages
due to their ability to phagocytose
what is the structure of canine mast cells and where are they found?
oval/irregular nucleus and granules
mainly located under epithelial surfaces
widely distributed throughout tissues of the body
what do granules in canine mast cells contain?
histamine
proteoglycans
neutral proteases
acid hydrolases
chemotactic factors
what triggers extracellular release of granules from canine mast cells?
triggered by physical, chemical, heat, trauma, toxins or immune mechanisms via antigen specific IgE binding
where do mast cells reside?
in tissues waiting for stimuli
what does the release of chemotactic factors do?
call more cells to help with protection
what are the functions of mast cells?
immune sentinel cells which respond directly to pathogens
send signals to other tissues to modulate both innate and adaptive immune responses
direct response = release of granules
whats the mean age for MCT in dogs?
7.5-9 years old
what triggers mast cell tumour mutation?
multifactorial but breed predisposition indicates possible genetic underpinning
what type of mutation can canine mast cells have?
c-kit mutations
what is KIT?
cell surface receptor expressed by normal and neoplastic mast cells
what is KIT encoded by?
proto-oncogene c-kit
what happens when KIT is stimulated by stem cell factor?
tyrosine-kinase activity
what is KIT involved in?
many activities including proliferation and survival
what is the structure of KIT?
two halves - one inside the cell and the other half outside the cell
what do the two arms of KIT do?
look for the lignin stem cell factor which triggers the cell to grow and survive
what happens when there is a mutation in the KIT receptor?
cells continue to proliferate and don’t die
where does the c-kit mutation in MCTs focus?
mutations predominantly focus on econ 11 - corresponds to the juxta membrane region of the protein
what type of mutation is c-kit mutation?
mainly internal tandem duplications and deletions
what are internal tandem duplication and deletions?
when a segment of DNA inside a gene is copied and inserted right next to the original segment
what does c-kit mutation cause?
causes the receptor to be active all the time whether the receptor mutation is inside the cell/outside the cell or struggling to find the lignin
what happens if mutations are in the juxta membrane regions?
may affect the localisation of receptor at the mast cell surface
other than genetics what else could cause MCT?
some cases are associated with chronic cutaneous inflammation
limited evidence of a possible viral trigger
environmental triggers may be possible
where are cutaneous MCTs most present?
the trunk and extremities and less common on the back and tail
what is the trunk?
abdomen/mid section
what is a well differentiated MCT?
tend to be solitary
rubbery 1-4 cm
slow growing
better prognosis
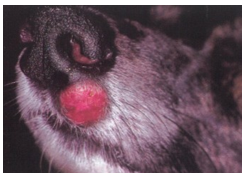
what is an intermediate MCT?
subcutaneous
soft/fleshy on palpation
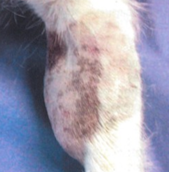
what is a poorly differentiated MCT?
rapid growth
ulceration
may give rise to small satellite nodules
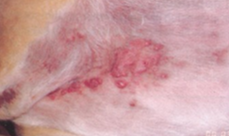
do satellite nodules have good prognosis?
no weak prognosis
what are the histological findings of grade 1/well differentiated MCT?
normal tissue cells still present
lots of undyed areas due to tissue organisation still intact
shape of mast cells are uniform and round
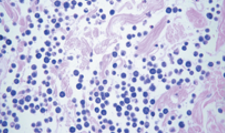
what are the histological findings of grade 2/intermediate MCT?
mast cells are disordered with different shapes
pleomorphic
tissue structures are being lost
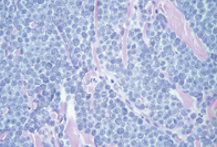
what are the histological findings of grade 3/ poorly differentiated MCT?
lots of oval shaped mast cells
limited normal cells left
mast cells are multi nucleated

what are the factors that can influence the prognostic outcome?
histological grade
clinical stage
location
clinical appearance
growth rate
presence of systemic paraneoplastic signs
breed
what areas are harder for prognosis and delay treatment?
tumour in nail bed, oral cavity, inguinal, perineal and mucocutaneous areas = worse prognosis and harder for owners to spot so delay treatment
what symptoms are associated with poorer prognosis?
ulcerations, erythema (reddening) or pruritus
what does growth rate relate to in terms of tumour?
tumour volume/duration
what tends to happen to spreading of cancer cells when the cancer gets bigger?
larger the tumour gets the more tumour cells being spread into the blood but also small tumour can be very aggressive
what are the treatment options for MCT?
surgery
radiotherapy
chemotherapy
other treatment modalities
same for most tumour types
when would surgery be performed?
treatment for well-differentiated and intermediate tumours
when surgery is performed on poorly differentiated tumours what are the outcomes?
used to alleviate symptoms (palliative) due to spreading but surgery can’t target all
potentially curative if tumour is small and there is no metastatic disease
what is de-bulking surgery?
reducing size of tumour
what needs to happen to get a curative response in surgery?
larger margin and some of healthy cells removed as well
what is a clean margin?
all tumour cells removed
what places are hard to perform surgery?
nasal as can cause more problems
when is radiotherapy used?
used when complete surgical resection is not feasible
what are the three types of radiotherapy?
X rays, gamma rays and electrons
how does radiation work?
works from outside of the tumour in
radiation causes DNA damage which leads to cell death during cell division
why does radiation work on cancer cells?
healthy cells can repair DNA but tumour cells are growing too quickly to have time to repair DNA and prevent death
what is used during radiotherapy to prevent damage to healthy cells?
lazers are used to map radiation to limiting hitting healthy cells an causing normal cell death
when is chemotherapy used?
disseminated
nonresectable
high grade tumours
how is chemotherapy administrated?
oral
intravenous
intra-cavity
intra-lesion
subcutaneous
when can chemotherapy be given?
neo-adjuvant - pre-surgery
adjuvant - on its own or post surgery
what are the two chemotherapy types?
cell cycle non-specific
cell cycle specific
what is cell cycle non specific?
disrupts the DNA double helix until tumour cells can’t repair and die
what is an example of cell cycle non-specific?
alkylating agents
what is cell cycle specific chemotherapy?
interferes with spindle formation to prevent DNA from being separated equally = cell death
during mitosis
what is the common outcome of chemotherapy?
cell can no longer divide = cell death
are healthy cells affected during chemotherapy?
yes and that is why there are side effects but healthy cells can repair themselves more easily due to growing more slowly
what do tyrosine kinase inhibitors do and what are the consequences?
target c-kit receptors and can kill the tumour but not fully curative
cancer must be in place to work and can cause gastrointestinal issues when killing mast cells which degranulate and empty contents into body
what happens when mast cell tumour cells release granules?
delayed wound healing (histamine suppression on fibroblasts and kertainocytes)
Local haemorrhage
gastrointestinal ulceration (due to vascular damage, excessive acid production and hyper motility)
anaphylactic shock (caused by sudden release of histamine)
what are the other treatment modalities/paraneoplastic treatments for MCT?
corticosteroids - control inflammation and palliative
H- 2 blockers - H 2 receptors antagonists used to mitigate the effects of histamine release