Unit 1 Summer Test
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
Saturated fatty acid
solid
Common in plants
Contributes to heart disease
Way more hydrogen than unsaturated
Unsaturated fatty acid
liquid at room temp
Double bonds in carbon chains
Less hydrogen
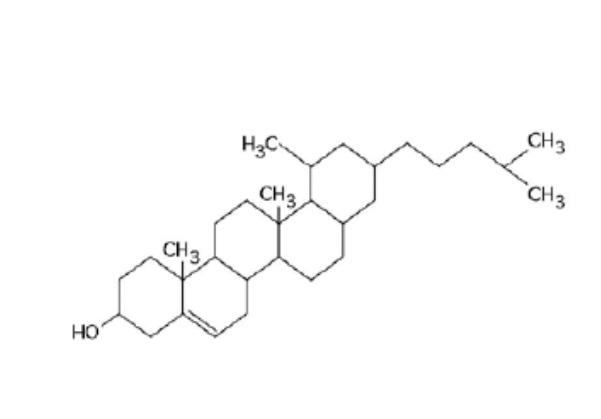
Structure of steroid
Synapse of impulses traveling (nerve)

Carboxyl group
COOH in organic acid
Can amino groups make an organic molecule less acidic
Yes
what constitutes an organism as being successful in evolutionary terms?
a particular species survives only a short period of time but leaves offspring that reproduce
What is natural selection
The process by which an organism that are better suited to their environment tend to survive and produce more offspring
Are chloroplasts part of the endomembrane system
No
Are DNA, starch, chitin, and cellulose polymers?
Yes
-polymers are linked with monomers
Lipids
fats
-macromolecule
Not soluble in water
Smooth ER function
Detoxification of poison and drugs
Lysosome function
Breakdown ingested substances
Hypotonic solutions
water moves into cell
Results in swelling
Hypertonic solutions
solute concentration is greater than that inside the cell, cell loses water
Cell bursts/shrinks
Isotonic solution
There is equilibrium
Nucleotide composed of:
Nitrogen base (ATGC), phosphate group, and a Penrose sugar
What are plant walls composed of
Cellulose
How do amino acids differ from each other
Side chains (r groups) attached to a carbon
Cell membranes
major structural component in phospholids and proteins
Small and hydrophobic molecules easily pass through
Water passes through aquaporins within
Proton pumps increase electrochemical gradient across membrane
What are the 4 elements that make up 96% of the body
Oxygen, carbon, nitrogen, hydrogen
What is the pH when the concentration of H+ ions and OH- ions in the water r in equilibrium
7 (pure)
What are two functional groups always found in amino acids
Carboxyl and amino
What can form polymers by dehydration reactions
Hydroxyl and Carboxyl groups
What assembles polymers
Dehydration reactions
Hydrolysis
Breaks down polymers by adding water
What is required when binding two amino acid molecules to make lg molecule
Release of water molecule
What are made from subunits by dehydration reactions
Proteins and polysaccharides and triglycerides
Ex. Of disaccharide
Lactose
Ex. Of monosaccharide
Fructose and carbs