Rad Bio Midterm
1/175
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
176 Terms
Who discovered evidence of radioactivity? (burn on his chest)
Antoine Henri Becquerel
Who first researched therapeutic effects? Defining Therapeutic ratio?
Clauddius Regaud
What stage(s) does HRR occur?
G2 and S only
What did Henri Coutard contribute in understanding fractionated treatments?
Efficacy (???) dependent on dose, treatment time, and number of sessions, and not time of treatment
What did Bergonie and Tribondeau report?
Radiosensitivity is related to mitotic activity
What did Coutard say about dose per fraction and tissue reactions?
“reducing dose per fraction, it is possible to delay tahe time to normal tissue reactions and to deliver higher doses to the tumor over longer times”
basically tumor control can be maintained while sparring healthy tissue if you reduce dose per fraction
What is the difference between scattered photon and characteristic photon through th ephotoelectric effect?
There is no scattered photon… the characteristic photon is ejected when a outer shell electron transisitons to fill the gap left by the ejected electron
What happens to the charactersitic photon produced via photoelectric effect? (where is it absorbed, bio consequence?)
absorbed locally with little biological consequence
Is compton Scattering dominant inair, water, or tissue?
same for all
What electrons are involved in Compton scattering?
Higher orbital, they are more loosely bound
What is produced in Compton scattering?
Recoil electron and scattered photon
What is the “Critical Target” in regards to direct and indirect action?
DNA
Through what kind of interactions are target atoms ionized/excited in Direct damage?
Coulomb interactions
How does Indirect action cause damage to target?
causes “free radicals” by interacting with molecules close to DNA which then interact with DNA
about what percentage of damage is caused by indirect action from X and gammas?
60-80%
What is the most common molecule ionized via indirect action that goes on the cause damage?
H2O
What is a “free radical” and why are they so chemically reactive?
atoms/molecules that carry and unpaired orbital electron - NOT charged.. unpaired electron makes them unstable
What process causes free radicals?
radiolysis
What is the lifetime of free radicals? Compared to typical time of completed reactions?
free radical lifetime - 10-6 seconds
reactions completed 10-12 to 10-9 seconds
“Radical effect can be modified by chemical interactions” … How?
radical scavengers - glutathione, thiols
oxygen mimetics - nictinomide, SOD
hypoxic radioresistance - tirapazamine
What is the most radiosensitive biomolecule?
DNA dummy
How do we know that DNA is the most radiosensitive?
This test showed us that irradiating cytoplasm was ineffective in cell death. but DNA caused cell death.
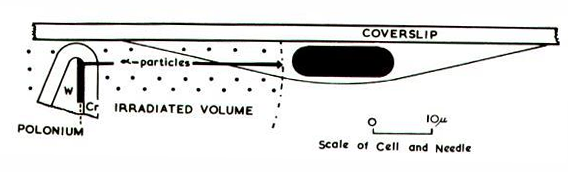
Theres a shit load of evidence for chromosomal DNA as principal target for cell killing….
yeah….
what is the caveat of DNA as principle target for cell killing?
“bystander cell killing”
Locally Multiply Damaged Sites (LMDS) a.k.a…..
clustered lesions
What is considered a lethal lesion?
(unrepaired) DSB
How can we measure the DNA damage?
Pulse Field Gel Electrophoresis (PFGE) - “molecular sieving” of damaged DNA in agarose
Single Cell Gell Electrophoresis - Comet
Limitations of PFGE?
Low resolution assay - ONLY GOOD FOR HIGH DOSE EXPOSURES
large cell sample required
no cell cycle discrimination
LIMITED CLINICAL APPLICATIONS
Benefits of Single Cell gel electrophoresis
Provides cell cycle info (based on dye fluorescence intensity)
can determine single/DSB (neutral pH for DSB, alkaline for SSB)
Greater clinical application - need less cells, measures tumor hypoxia
better low dose resolution (TIME CONSUMING)
4 possible repair pathways for chromosomes after dose?
no repair - possible lethal lesion
Reconstitute (repair) normally - no biological consequence
Reconstitute with poor fidelity - MISREPAIR
Reconstitute abnormally - MISJOINING
Difference between misrepair and misjoining? (in terms of cell death)
Misrepair is NON LETHAL- possible carcinogenetic event
Misjoining MAY BE LETHAL - causes aberrations…
Aberrations caused by misjoining?
Dicentric and Centric rings aberrations, deletions, and translocations
What does Giesma (G) banding do to DNA?
stains them (A-T preferentially) to detect changes in quantity and structure of chromosomes
looks for aberrations (Dicentric and centric rings, acentric fragments. NO TRANSLOCATIONS, insertions, or complex rearrangements)
What is the hallmark aberration of ionizing radiation exposure?
reciprocal translocation
What does Chromosome painting do/detect? (FISH)
Detects all!
3 main types of lethal DNA damage? (chromosome and chromatid)
chromosome: dicentric and ring structure
chromatid: anaphase bridge
common non-lethal rearrangements?
translocations (radiation biomarker)
interstitial deletions
What happens to number of lethal aberrations after exposure? (number up or down?) What does this mean for measurement of dose?
number down with time post exposure… So dose scoring is underestimated with time after exposure
What is Dicentric aberration? (chromosome aberration)
DSB on two separate chromosomes → one chromosome has 2 centromeres + acentric fragment
what is ring structure aberration? (chromosome aberration) When does it occur?
DSB in each arm of chromatid in G1
interaction between two chromosomes (CHECK THIS,,, I edited this retrospectively from a later slide)
→ two DSBs rejoin to form a ring + fragment
What is anaphase bridge? (chromatid aberration)
one DSB in both chromatid of same chromosome (two total DSBs)
→ ring like structure + fragment
non-lethal (stable) aberrations? (3)
viable mitotic events
translocations
micronuclei events → one DSB
Timeframe for viable mitotic events? (like translocation and deletions? idk what this is)
can persist for years, lymph progenitors and stem cells sustained
ACCURATE estimate of exposure dose
What are translocations?
RADIATION BIOMARKERS
whole-body biological dosimeter as low as 25cGy
2 types of translocations?
symmetrical reciprocal → 1 break in 2 different pre-replication chromosomes
asymmetrical → YO THESE ARE THE SAME ON THE SLIDE WTF
When analyzing translocation numbers from different dose strengths, what is important to consider?
Loss in biological dosimetry long after time of exposure? More pronounced for higher doses (1Gy used as example for acute dose)
What is the LQ response a measure for?
dose response for DSB chromosome aberrations, LOW LET..
What are the two main components of the LQ response curve?
Linear portion at lower dose is result of single events causing 2 breaks, or isolated events
Quadratic section → higher doses shows probability of interaction proportional to (dose)2 (Because rings and dicentric aberrations are caused between tw0o chromosomes)
What does the LQ response show in regards to dose?
Fewer aberrations at lower dose rates
How is recovery at the cellular level assessed?
By changes in survival response using clonogenic assays
3 ways radiation damage is operationally characterized?
Lethal damage - irreparable, no chance of survival
Potentially Lethal Damage (PLD)
Sub-lethal Damage (SLD)
Describe PLD
Potentially Lethal Damage → from a single dose. Survival chances modified by post radiation conditions (e.g. restricting/preventing cell division)
Describe SLD
Sub-lethal Damage → from 2 doses. . Elkind repair (split dose recovery): repair of sub lethal lesions during time between doses. without added damage, 2 hour repair, with more damage, 6-8 hours repair
What kind of damage can be modified by post-irradiation environmental conditions?
PLD
What kind of tumors are most often demonstrate PLD repair?
LARGE tumors
because they are generally non-proliferating, they are more radioresistant
How can Sub Lethal Damage (SLD) turn into lethal lesion?
if more SLD occurs from subsequent exposure, 2 SLD lesions combine producing irreparable lethal lesion
What is basis for fractionation for sparring normal tissue?
SLD repair. Fractionating spares damage to late responding normal tissue
How much time is adequate fractionation for full SLD repair of normal tissue?
Daily fractionation
What part of LQ model is SLD? What part of α/β?
Shoulder of curve (indicates repair) … β portion!
What does a large shoulder of survival curve say about α/β?
Small α/β, because larger β component
reflective of reparability and dose rate sparing
How does dose rate affect survival for normal tissue?
SLD repair can take place during treatment, INCREASING survival
As dose rate is reduced, exposure time increases → reducing possibility for lesion interactions
What kind of repair is modeled by the split-dose experiment?
Elkind repair
What kind of plot is LQ curve on?
Log-linear
What is DQ and D0 of LQ curve?
DQ is size of shoulder. D0 is slope.
What does α/β say about the LQ curve?
describes shape
What protein determines radiosensitivity?
ATM (and ATR, DNA-PK)
If deficient, you are more radioSENSITIVE
What protein is recruited by MRN to initiate DSB repair?
ATM
what are the 2 main DNA DSB repair pathways?
Non-homologous End Joining (NHEJ)
Homologous recombination repair (HRR)
Which DNA DSB repair pathway is simpler?
NHEJ
Which DNA DSB repair pathway is more error prone?
NHEJ
Main action of HRR?
Employs undamaged sister chromatid to promote repair
high fidelity repair
Main action NHEJ?
Just rejoin at the ends… no cleaning or anything (why its error prone)
What kind of DNA DSB repair do proliferating tumor cells prefer?
HRR, because it only occurs in S/G2 phase
What kind of DNA DSB repair do normal tissue prefer?
NHEJ
Which cell cycle phase is most radiosensitive?
Mitosis: chromosome condensation, lack of HRR
At what points in the cell cycle does ATM signal repair and arrest?
Checkpoints: G1 (gap 1), S, and Two G2 (gap 2)
Cancer is characterized by__________?
aberrant cell cycle activity
Chromosome breaks occurs where? (cell cycle)
mitosis
Drugs that inhibit repair target which DNA DSB repair pathway?
HRR
What inhibitor induces cell cycle checkpoints?
CDK inhibitor p21 (regulated by p53)
can arrest cell cycle following damage
Which part of cell cycle is critical for carcinogenesis?
G1-S
Positive regulation of cell cycle is dependent on what paired proteins?
cyclin and cyclin dependent kinase (CDK)
What paired proteins (gene encoders) trigger G1/S transition? How is this regulated?
cyclin D and CDK4 → phosphorylates RB → triggers G1/S
negative regulation by CDKN2A
Cyclin D/CDK4 phosphorylate _____ to trigger G1/S transition?
is this positive or negative regulation by CDKN2A?
Retinoblastoma, Rb… negative regulation
What inhibitor is highly mutated in cancer, preventing cell cycle arrest?
P53
What is retinoblastoma? what does it lead do? How can it proliferate in regards to CCND/CDK4?
Key cancer suppressant gene…
leads to hyper-phosphorylation and cell cycle progression
Can proliferate independently of CCND CDK4
How is cell cycle control disrupted? (basically, how does cancer form? 3 things)
Loss-of-function mutations in either RB1 or CDKN2A (p16)
Gain-of-function mutations in Cyclin D’s (CCND1,2,3)
CDK4,6 constitutively phosphorylated and RB inactivated
What genes (tumor suppressants) does cancer preferentially delete to continue proliferating?
p15 (encoded by CDKN2A), p16 (encoded by CDKN2B)
What is most frequently deleted locus in cancer?
CDKN2A
Second most frequently deleted locus in cancer?
Cyclin D1 associated genes
What does oncogene do?
Gene amplifications (gain of function)…. drives proliferations of CCND’s
Can induce Apoptosis
What is most radioresistant phase in cell cycle?
S phase → Specifically LATE S … followed by early S phase
Why is Late S more resistant than others?
sulfhydryls in S phase, Repair, checkpoints, and DNA organization
How to overcome s-phase radioresistance?
High LET particles
Which checkpoint is ATM dependent?
Early G2
What checkpoint arrests cells that were irradiated in G1 & S?
Sinclair checkpoint (Late G2 checkpoint)
Why are tumors slow to regress?
delayed cell death (abortive colony… old paradigm)
What does a loss of clonogenic potential cause?
Cell death
4 methods of cell death after irradiation?
post mitotic inactivation
pre-mitotic interphase inactivation
apoptosis, autophagy, necrosis
replicative senescence
What is the mechanism of cell death for solid tumors?
Post mitotic inactivation… a.k.a. chromosome/chromatid aberrations