Concept 8.4: Enzymes speed up metabolic reactions by lowering energy barriers
1/18
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
Spontaneous reaction
A reaction that does not need added energy but can be slow enough to be imperceptible
Hydrolyzing sucrose to glucose and fructose with water can take years without a catalyst
Catalyst
A chemical agent that speeds up a reaction without being consumed by the reaction
Enzyme
A macromolecule (typically protein) that acts as a catalyst to speed up a specific reaction
These lower the activation energy barrier enough for the reaction to occur at moderate temperatures while being reusable
Names typically end in -ase for a specific reaction and substrate
Chemical bonds
These break and form in a chemical reaction
Breakage requires excessive contortion before absorbing energy from its surroundings
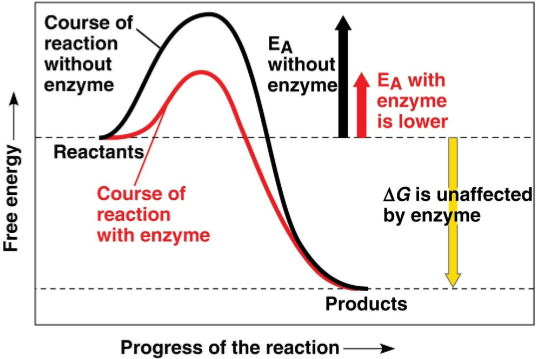
Activation energy (EA)
The initial energy needed to break the bonds of the reactants, often seen as heat from the surroundings
Instability occurs when enough energy is absorbed to start the reaction
Provides a barrier that determines the rate of spontaneous reactions
Only changes speed and not overall effect of the reaction
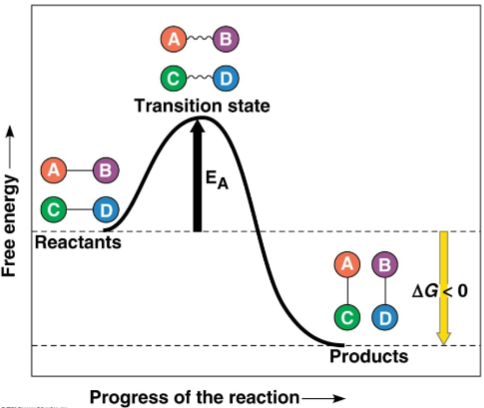
Exergonic reaction
A reaction that releases more energy than was initially invested through the formation of new bonds
These new bonds increase stability
Catalysis
The process by which a catalyst selectively speeds up a reaction without itself being consumed
Avoids the excessive use of heat to speed up reactions which can cause denaturation
Substrate
The reactant that an enzyme acts on through catalytic activity for a conversion to products
Typically held in the enzyme’s active site by weak bonds, such as hydrogen bonds
Can be reoriented, stretched, or placed in a microenvironment to favor the reaction
Rates of reaction can be increased with higher concentrations until complete enzyme saturation
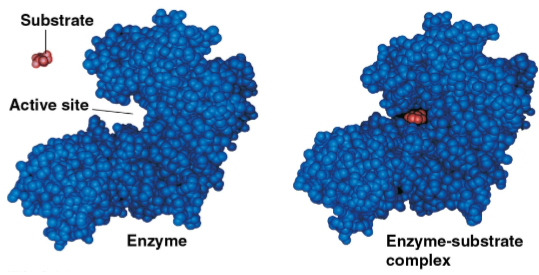
Active site
The region on the enzyme that binds to the substrate, fitting the specific shape of the substrate
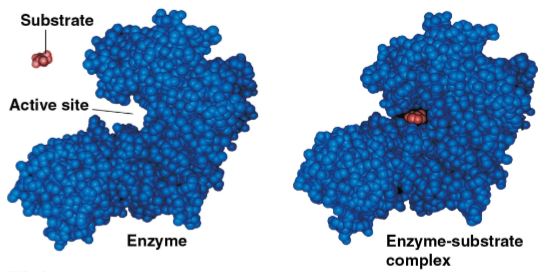
Induced fit
The slight change in shape of the enzyme like a handshake that results from chemical interactions on the substrate and active site
This ultimately enhances catalysis of the reaction
Optimal conditions
Conditions that are most conducive to an enzyme’s function, mainly defined within a range of temperature, pH, and chemicals that influence the enzyme
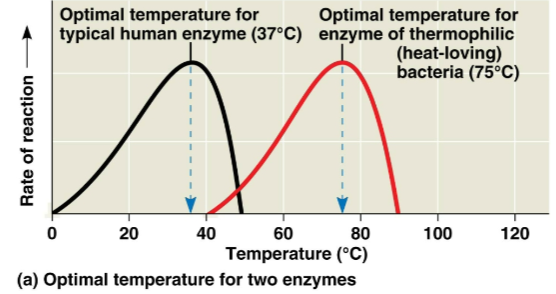
Optimal temperature
The temperature at which an enzyme catalyzes its reaction at the maximum possible rate, increasing with increasing temperature until it is met and begins to drop and denature
Typically defined by the environment in which it functions — human enzymes adapt to human body temperature, or around 37 degrees C
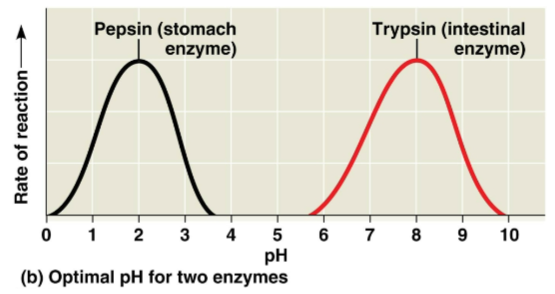
Optimal pH
The pH at which an enzyme is typically active, usually dependent on the environment at which it functions
Pepsin in the stomach has an optimal pH of 2
Cofactors
Nonprotein helpers that bind to the enzyme permanently or reversibly with the substrate
Inorganic includes metal atoms such as zinc, iron, and copper in ionic form
Organics are called coenzymes, found in vitamins or made from their raw materials
Coenzymes
Organic cofactors that include vitamins or their raw materials as helpers to enzymes
Enzyme inhibitor
A chemical that inibits the action of specific enzymes
Inhibition with covalent bonds to the enzyme are usually irreversible
Weak interactions can be reversible and are used in most cases
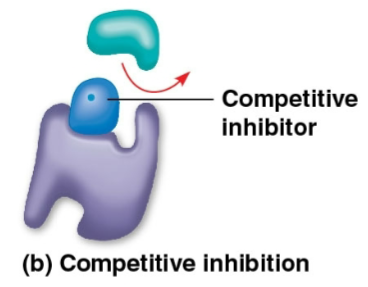
Competitive inhibitors
Inhibitors that closely resemble the substrate of an enzyme and can bind to the enzyme’s active site, thus reducing enzyme productivity due to blockage
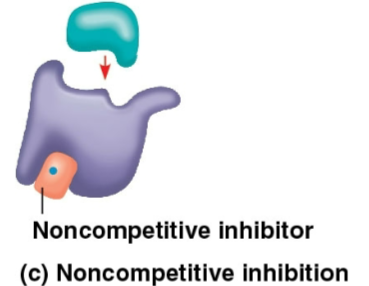
Noncompetitive inhibitors
Inhibitors that bind to another part of the enzyme away from the active site
These cause the enzyme to change shape and makes the active site less effecive at catalyzation
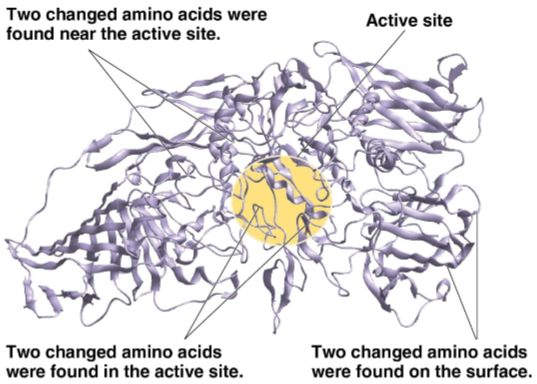
Genes
The encoding method for enzymes; mutations in these can lead to a positive or negative change in the enzyme’s amino acid composition and thus new activity or substrate specificity