chem final (gases)
0.0(0)
Card Sorting
1/8
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
1
New cards
Kinetic Molecular Theory (KMT)
the behavior of ideal gas particles
2
New cards
1/5 KMT
tiny particles in large space
3
New cards
2/5 KMT
random constant motion
4
New cards
3/5 KMT
elastic collisions
5
New cards
4/5 KMT
no attractive forces
6
New cards
5/5 KMT
directly proportional to temp
7
New cards
Ideal Gas
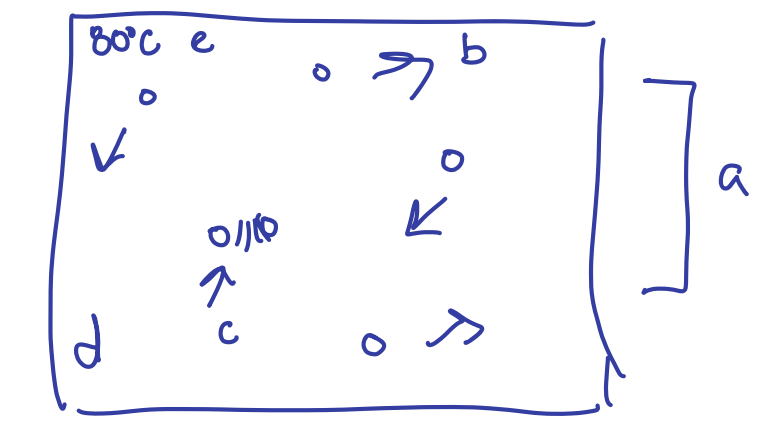
8
New cards
avogadro’s hypothesis
explains how STP conditions are the same for every ideal gas and if pressure, volume and temperature are constant then moles are too
9
New cards
STP
standard temperature and pressure