CHEM 1030: Exam 2
1/117
Earn XP
Description and Tags
Chapters 4-7
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
118 Terms
C₂H₃O₂⁻
Acetate
CO₃⁻²
Carbonate
HCO₃⁻
Hydrogen carbonate or bicarbonate
OH⁻
Hydroxide
NO₂⁻
Nitrite
NO₃⁻
Nitrate
CrO₄⁻²
Chromate
Cr₂O₇⁻²
Dichromate
PO₄⁻³
Phosphate
HPO₄⁻²
Hydrogen phosphate
H₂PO₄⁻
Dihydrogen phosphate
NH₄⁺
Ammonium
ClO⁻
Hypochlorite
ClO₂⁻
Chlorite
ClO₄⁻
Perchlorate
MnO₄⁻
Permanganate
SO₃⁻²
Sulfite
HSO₃⁻
Hydrogen sulfite or bisulfite
SO₄⁻²
Sulfate
HSO₄⁻
Hydrogen sulfate or bisulfate
CN⁻
Cyanide
O₂⁻²
Peroxide
ClO₃⁻
Chlorate
hemi-
½
mono-
1
di-
2
tri-
3
tetra-
4
penta-
5
hexa-
6
hepta-
7
octa-
8
Ionic bonds
transfer of electrons between metals and nonmetals
in ionic bonds the metal atom becomes a
cation
In ionic bonds the nonmetal becomes a
anion
Covalent bond (molecular bonds)
sharing of electrons between two or more nonmetals
Ionic or molecular compound?
CaF₂
Ionic
Ionic or molecular compound?
F₂O
Molecular
Ionic or molecular compound?
Al₂O₃
Ionic
Ionic or molecular compound?
HCl
Molecular
Empirical formula definition
relative number of atoms of each element in a compound (simplest ratio)
Molecular formula definition
gives the actual number of atoms of each element in a molecule of a compound
What is the empirical formula?
C₆H₆
CH
What is the empirical formula?
P₅H₁₀
PH₂
What is the Electron Geometry?
Electron groups: 2
Linear
What is the Electron Geometry?
Electron groups: 3
Trigonal Planar
What is the Electron Geometry?
Electron groups: 4
Tetrahedral
What is the Electron Geometry?
Electron groups: 5
Trigonal bipyramidal
What is the Electron Geometry?
Electron groups: 6
Octahedral
What is the Molecular Geometry?
Electron Groups: 2
Bonding Groups: 2
Lone Pairs: 0
**pay attention to picture
Linear
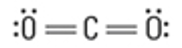
What is the Molecular Geometry?
Electron Groups: 3
Bonding Groups: 3
Lone Pairs: 0
***select one with picture
Trigonal Planar
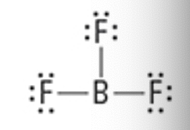
What is the Molecular Geometry?
Electron Groups: 3
Bonding Groups: 2
Lone Pairs: 1
***pay attention to picture to0
Bent
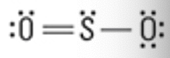
What is the Molecular Geometry?
Electron Groups: 4
Bonding Groups: 4
Lone Pairs: 0
***select one with pictures
Tetrahedral
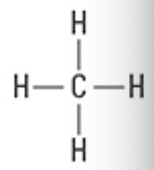
What is the Molecular Geometry?
Electron Groups: 4
Bonding Groups: 3
Lone Pairs: 1
Trigonal pyramidal
What is the Molecular Geometry?
Electron Groups: 4
Bonding Groups: 2
Lone Pairs: 2
***pay attention to picture too
Bent
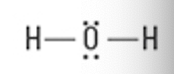
What is the Molecular Geometry?
Electron Groups: 5
Bonding Groups: 5
Lone Pairs: 0
***select one with picture
Trigonal bipyramidal
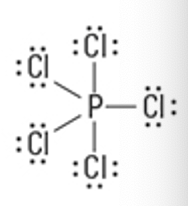
What is the Molecular Geometry?
Electron Groups: 5
Bonding Groups: 4
Lone Pairs: 1
Seesaw
What is the Molecular Geometry?
Electron Groups: 5
Bonding Groups: 3
Lone Pairs: 2
T-shaped
What is the Molecular Geometry?
Electron Groups: 5
Bonding Groups: 2
Lone Pairs: 3
***pay attention to picture
Linear
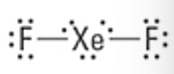
What is the Molecular Geometry?
Electron Groups: 6
Bonding Groups: 6
Lone Pairs: 0
***select one with picture
Octahedral
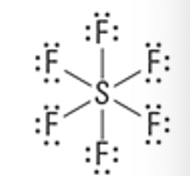
What is the Molecular Geometry?
Electron Groups: 6
Bonding Groups: 5
Lone Pairs: 1
Square pyramidal
What is the Molecular Geometry?
Electron Groups: 6
Bonding Groups: 4
Lone Pairs: 2
Square planar
What bond angle does this geometry have?
Linear
180°
What bond angle does this geometry have?
Trigonal planar
120°
What bond angle does this geometry have?
Trigonal planar Bent
<120°
What bond angle does this geometry have?
Tetrahedral
109.5°
What bond angle does this geometry have?
Trigonal pyramidal
<109.5°
What bond angle does this geometry have?
Tetrahedral Bent
<109.5°
What bond angle does this geometry have?
Trigonal bipyramidal
120° (equatorial) & 90° (axial)
What bond angle does this geometry have?
Seesaw
<120° (equatorial) & <90° (axial)
What bond angle does this geometry have?
T-shaped
<90°
What bond angle does this geometry have?
Octahedral
90°
What bond angle does this geometry have?
Square pyramidal
<90°
What bond angle does this geometry have?
Square planar
90°
Symmetrical or asymmetrical?
Linear
symmetrical
Symmetrical or asymmetrical?
Trigonal Planar
symmetrical
Symmetrical or asymmetrical?
Bent
asymmetrical
Symmetrical or asymmetrical?
Tetrahedral
symmetrical
Symmetrical or asymmetrical?
Trigonal pyramidal
asymmetrical
Symmetrical or asymmetrical?
trigonal bipyramidal
asymmetrical
Symmetrical or asymmetrical?
octahedral
symmetrical
Symmetrical or asymmetrical?
seesaw
asymmetrical
Symmetrical or asymmetrical?
T-shaped
asymmetrical
Symmetrical or asymmetrical?
square pyramidal
asymmetrical
Symmetrical or asymmetrical?
square planar
symmetrical
H only needs __ electrons
2
Be only needs __ electrons
4
B only needs __ electrons
6
Given 2 electron groups and linear electron geometry what are the hybrid orbitals
2: sp
Given 3 electron groups and trigonal planar electron geometry what are the hybrid orbitals
3: sp2
Given 4 electron groups and tetrahedral electron geometry what are the hybrid orbitals
4: sp³
Given 5 electron groups and trigonal bipyramidal electron geometry what are the hybrid orbitals
5: sp³d
Given 6 electron groups and octahedral electron geometry what are the hybrid orbitals
6: sp³d²
A single bond has
__ 𝜎 bonds
__ π bonds
1 𝜎 bonds
0 π bonds
A double bond has
__ 𝜎 bonds
__ π bonds
1 𝜎 bonds
1 π bonds
A triple bond has
__ 𝜎 bonds
__ π bonds
1 𝜎 bonds
2 π bonds
Bonding molecular orbitals are designated by…
A) 𝜎* and π*
B) being italicized
C) 𝜎 and π
D) only 𝜎
E) only π
C
Antibonding molecular orbitals are designated by…
A) 𝜎* and π*
B) being italicized
C) 𝜎 and π
D) only 𝜎
E) only π
A
Formula for bond order
(# bonding electrons - # antibonding electrons)/2
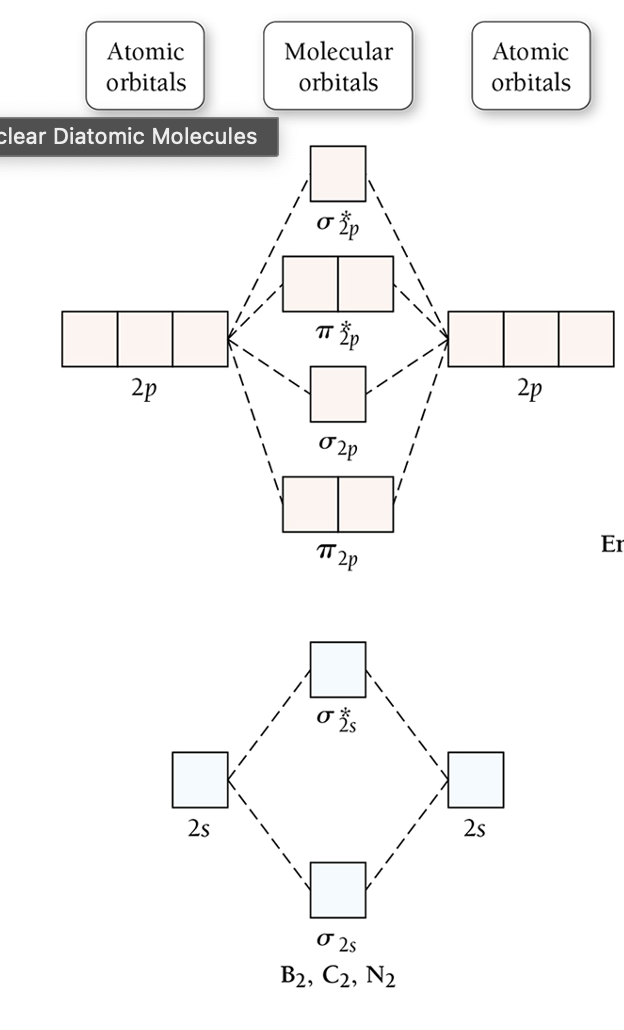
What is the molecular orbital energy ordering for B₂, C₂, N₂