ionic bonding
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
What is ionic bonding explain process
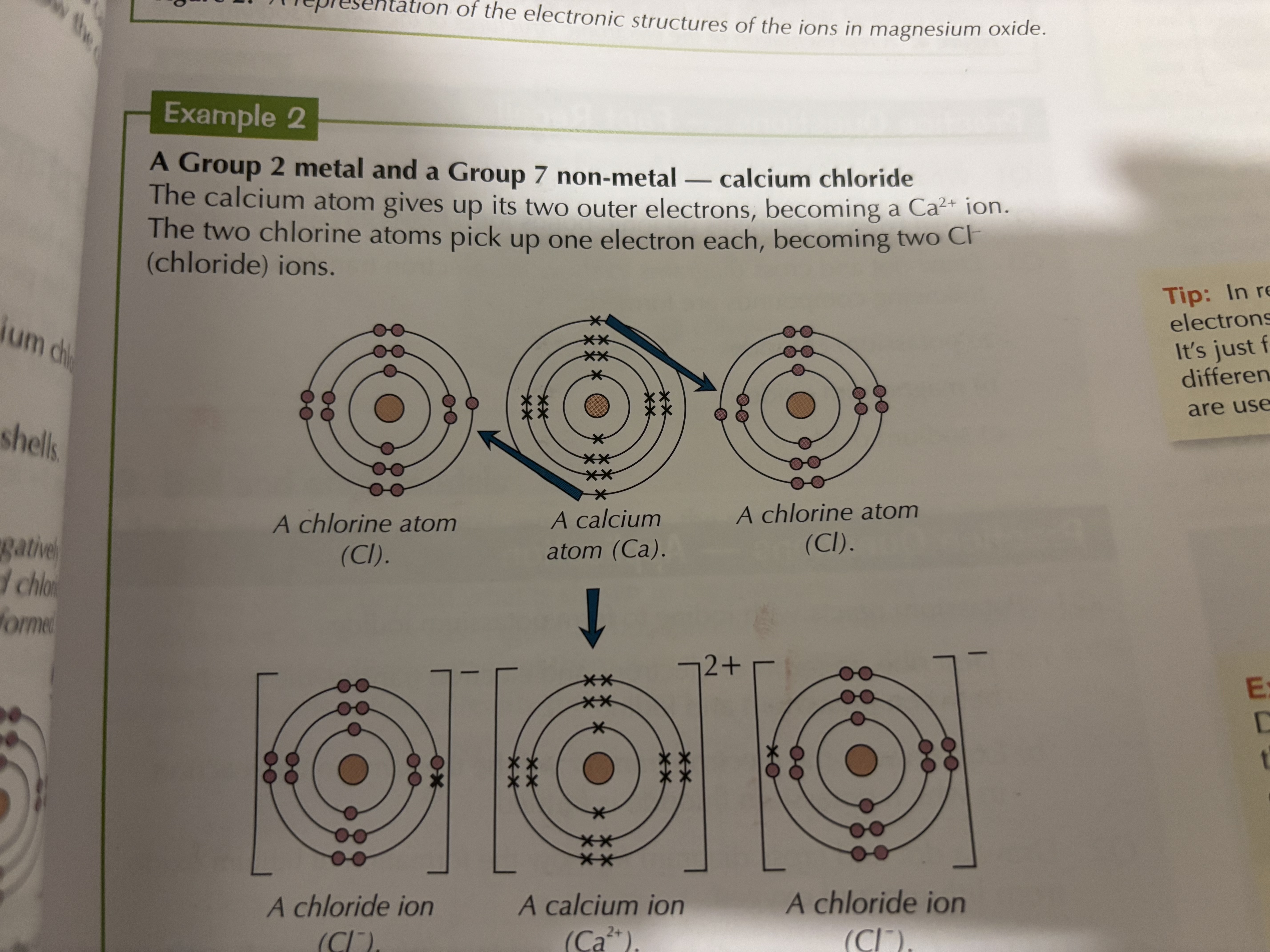
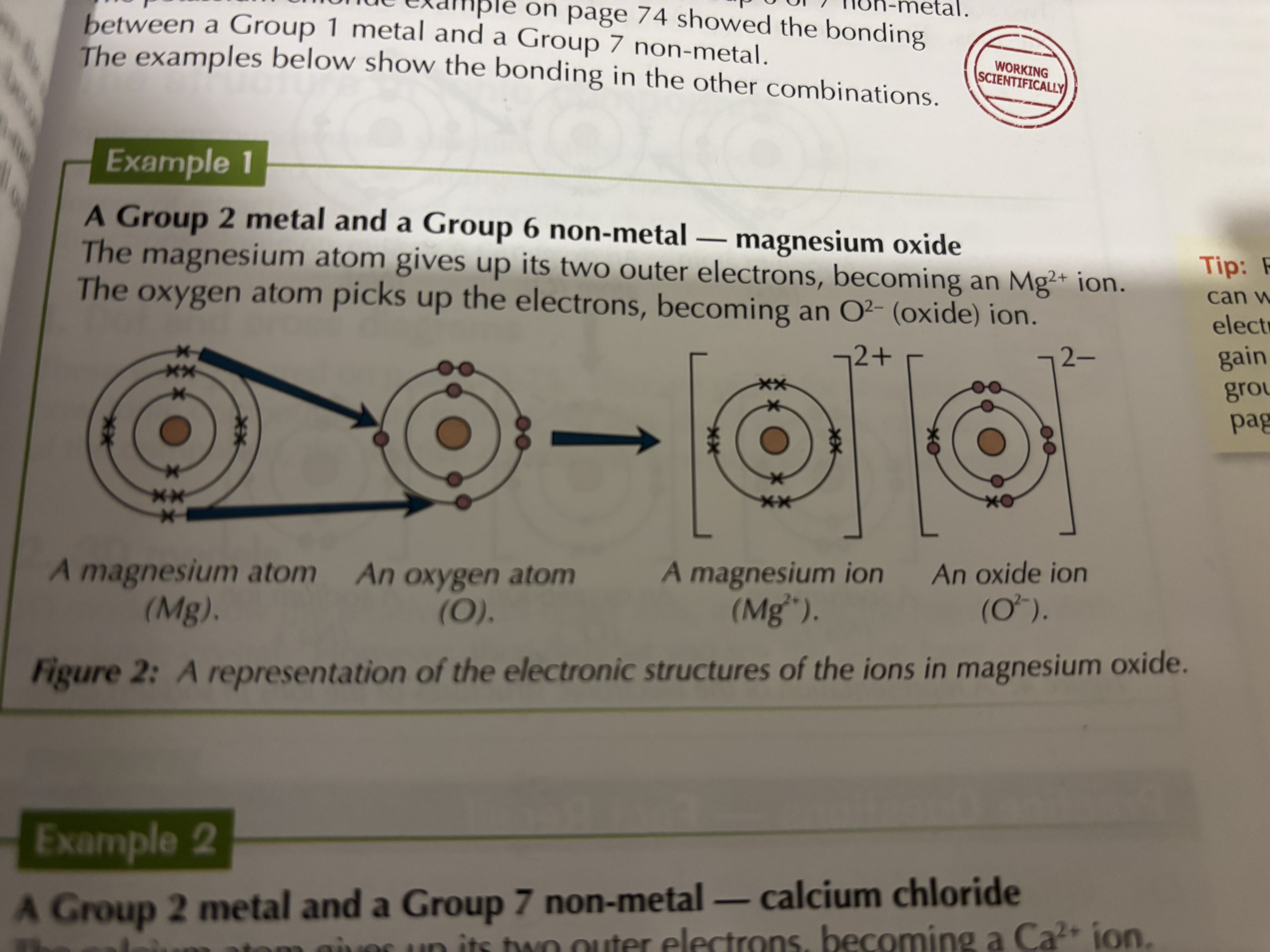
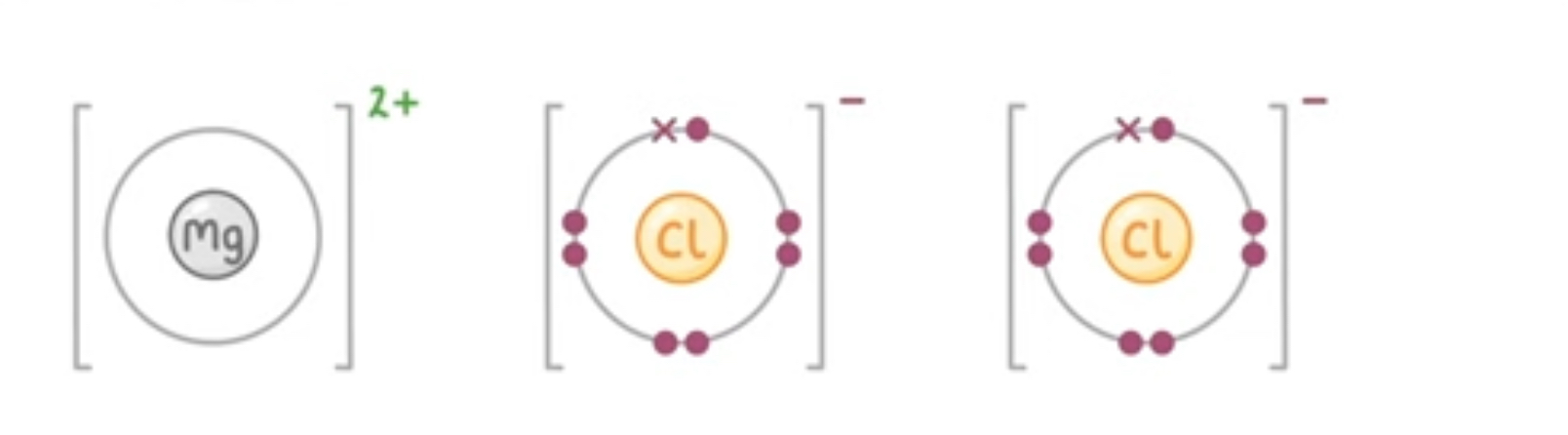
When metals react with nonmetals, electrons are transferred from the metal atoms to the non-metal atoms. The metal atoms lose electrons to become positively charged ions with a full shell of electrons. The non-metal atom gains electrons and becomes negatively charged ions with a full outer shell of electrons.
Extra note
What are the ions attracted to each other through
Two ions have opposite charges so they are attracted to each other through electrostatic forces
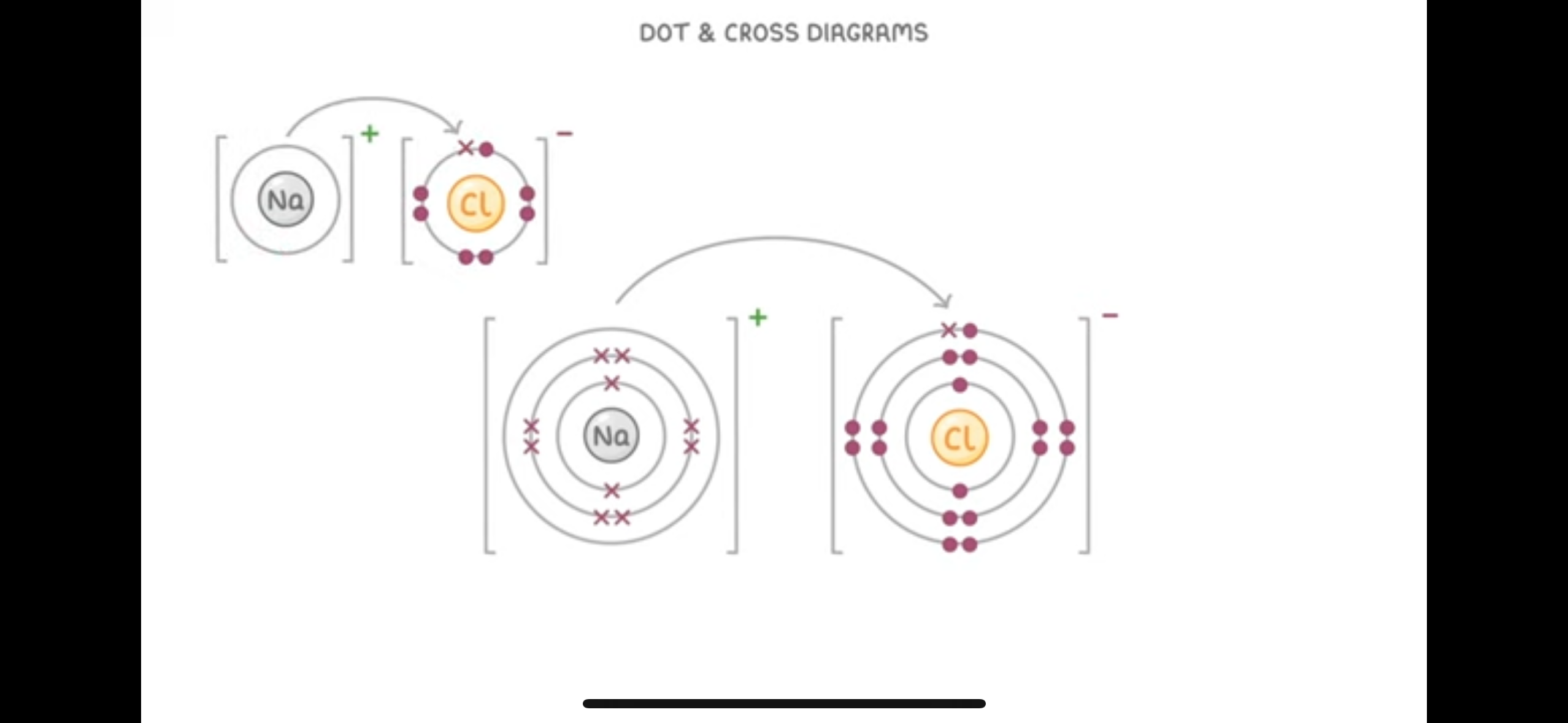
What are dot and cross diagrams used to show
What happens during ionic bonding. The electrons in one type of atom are represented by dot and the other represented using crosses
helps to clearly show the lose and gain of electrons
Dot and cross diagrams