Bronsted-Lowry Acids and Bases
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
What is a Bronsted-Lowry acid?
a species that donates a proton
What is a Bronsted-Lowry base?
a species that accepts a proton
What are conjugate acid-base pairs?
Contains 2 species that can be inter converted by transfer of a proton
What is the conjugate acid-base pairs for ethanoic acid?
H+(aq) and CH3COO-(aq)
What is the conjugate acid-base pairs for HCl?
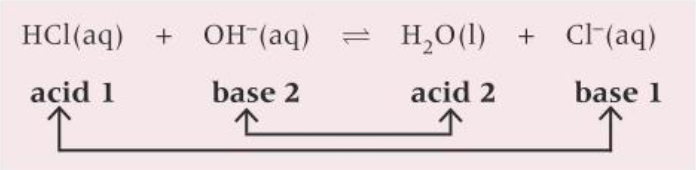
What are the conjugate base pairs for HCl in aqueous solution?
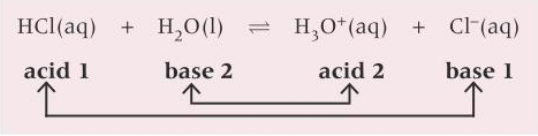
What are monobasic acids?
acids that have 1 hydrogen ion that can be replaced per molecule in an acid-base reaction
one mole of the acid dissociates to form one mole of H+
E.g HCl, and CH3COOH
What are dibasic acids?
acids that have 2 hydrogen ions that can be replaced in an acid-base reactions
E.g H2CO3
What are tribasic acids?
acids that have 3 hydrogen molecules per molecule that can be replaced in an acid-base reaction
E.g H3BO3
What are the ionic equations for the reactions between Mg and HCl/H2SO4?
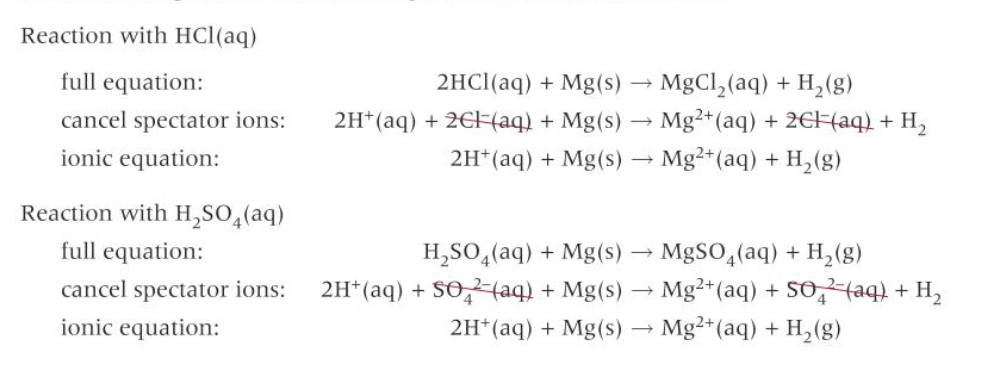
What is the ionic equations for the reactions between acid and metals?
2H+ + Zn → Zn3+ + H2
What is the ionic equation for the reactions between an acid and carbonate?
2H+ + CO32- → Cu2+ + H2O + CO2
What is the ionic equation for the reaction between and acid and base?
2H+ + MgO → Mg2+ + H2O
What is the ionic equation for the reaction between an acid and alkali?
H+ + OH- → H20