addition reactions
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
hydration
alkene reacts with water to form alcohol
hydration forms
Markovnikov
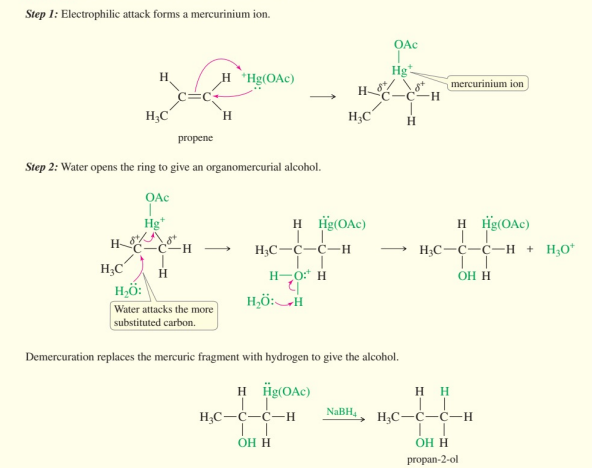
oxymercuration-demercuration steps
form mercurinium
water opens ring
demercuration replaces mercuric with alcohol via NaBH4
alkyloxymercuration-demercuration
alcohol nucleophilic attack to mercurinium
hydroboration-oxidation
alkene → anti-Markovnikov alcohol
hydroboration oxidation mechanism
peroxide effect
BH3 is e- deficient & adds itself to less substituted end to form radical
syn addition
halogen addition steps
form halonium
2nd halide ion attacks backside
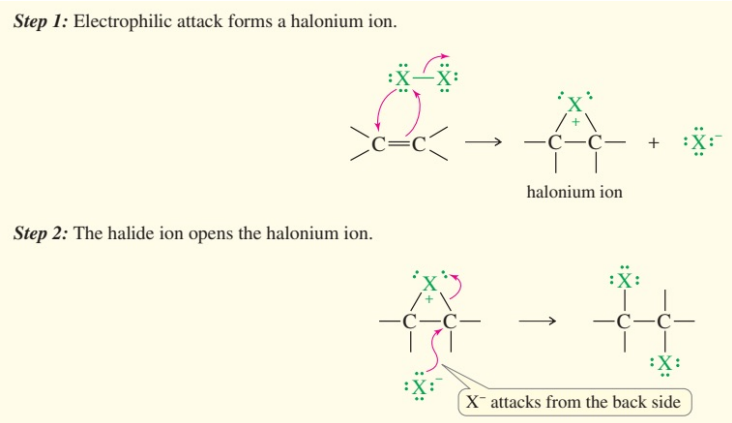
halogen addition is
anti addition
formation of halohydrin
alcohol & halogen on adjacent carbons
steps of halohydrin formation
halonium
H2O backside attack
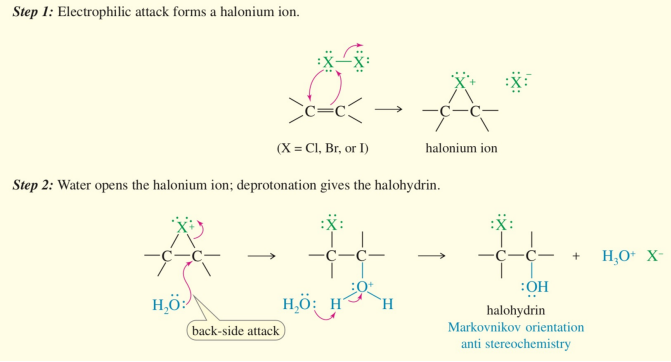
formation of a halohydrin is
anti-addition
markovnikov product
catalytic hydrogenation of alkenes
syn addition of H2 to alkene
carbene addition
form cyclopropane with alkene
simmons-smith addition
methylene iodide & to Zn(Cu)
epoxidation of alkene
alkene + peroxyacid to form 3 member cylic ether epoxide

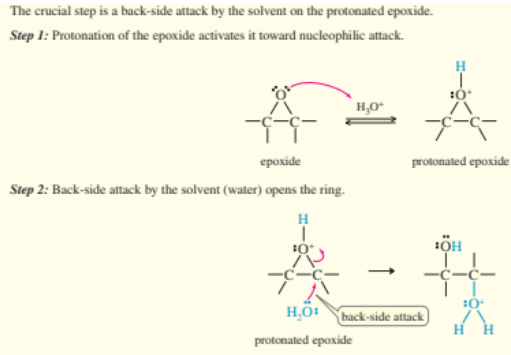
acid catalyzed opening of epoxides
slightly acidic solvent protonates epoxide to form glycol
acid catalyzed opening of epoxide steps
protonation of epoxide
H2O nucleophilic backside attack
deprotonation
syn dihydroxylation
add OH to same face of C=C via OsO4 OR KMnO4