Chemistry 11 - Chapter 4 (Classifying Chemical Reactions)
5.0(1)
Card Sorting
1/62
Earn XP
Last updated 1:32 AM on 2/21/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
63 Terms
1
New cards
What is chemistry?
The study of matter and its changes.
2
New cards
What are chemical changes?
Any change in which experimental evidence indicates that original substances (reactants) have been consumed and new substances (products) have been produced. A chemical change involves a reorganization of atoms, ions, or molecules by the breaking and reforming of chemical bonds.
3
New cards
What are the five different kinds of experimental evidence?
1. Noticeable energy change
2. Change in color
3. Formation of a gas in the absence of boiling
4. Formation of a precipitate from two solutions
5. Relatively difficult to reverse
4
New cards
Why do we balance chemical equations?
Because of Lavoisier’s Law of Conservation of Mass
5
New cards
Why is it helpful to classify reactions?
Classifying reactions helps us predict the products of chemical changes and predict whether or not a reaction is likely to occur.
6
New cards
What are the six major categories of chemical reactions?
1. Synthesis
2. Decomposition
3. Single replacement
4. Double replacement
5. Neutralization
6. Combustion
7
New cards
What is a synthesis reaction?
Two or more simple substances (elements or compounds) combine to form one more complex substance.
8
New cards
Are synthesis reactions endothermic or exothermic?
Exothermic, often accompanied by a significant release of heat and light.
9
New cards
What is the energy required to initiate a chemical reaction called?
Activation energy
10
New cards
When a metal oxide combines with water, what forms?
A base
11
New cards
When a non-metal oxide combines with water, what forms?
An acid
12
New cards
What is a decomposition reaction?
When a complex compound is broken down or decomposed into two or more simpler substances (element or compound)
13
New cards
Are decomposition reactions endothermic or exothermic?
Endothermic (reverse of synthesis reactions)
14
New cards
What do decomposition reactions generally involve?
They generally involve one of the following:
* An acid decomposing to form water and non-metal oxides
* A base decomposing to form metal oxides and water
* Ternary salts decomposing to form oxides of metals and non-metals
* An acid decomposing to form water and non-metal oxides
* A base decomposing to form metal oxides and water
* Ternary salts decomposing to form oxides of metals and non-metals
15
New cards
What is a combustion reaction?
The reaction of a hydrocarbon, carbohydrate, or alcohol reacting with oxygen gas to produce carbon dioxide gas and water.
16
New cards
Are combustion reactions endothermic or exothermic?
Exothermic, releasing a significant amount of energy in the form of heat, light, and/or sound
17
New cards
What is a single replacement reaction?
A reaction between a compound and an element so that the element replaces an element of the same type in the compound, resulting in a new compound and a new element.
18
New cards
What loses and what gains electrons in a single replacement reaction?
The thing doing the replacing loses electrons and the thing getting replaced gains electrons.
19
New cards
How do we determine if a single replacement reaction will proceed?
We must compare the chemical reactivity of the element to that of the element it will replace using the activity series table.
20
New cards
What is chemical reactivity?
The tendency of a substance to undergo a chemical change.
21
New cards
What is a double replacement reaction?
A chemical reaction between two compounds that trade cations or anions with one another.
22
New cards
What are the three categories of double replacement reactions?
1. Precipitation reaction
2. Neutralization reaction
3. Gas formation reaction
23
New cards
What is a precipitation double replacement reaction?
When two freely soluble salts trade partners, two new compounds are formed, one of which is a precipitate.
24
New cards
What is immediate evidence that a precipitation reaction has occurred?
The reaction ends with a solid suspended in solution.
25
New cards
How do we know which product forms a precipitate in a precipitation reaction?
Verify using the solubility table
26
New cards
What is a neutralization reaction?
When an acid and a base react to form a salt and water.
27
New cards
How do we test that a neutralization reaction occurred?
With a pH test or litmus paper to see that the solution in neutral (no more acid or base in the solution)
28
New cards
How do we know that the acid is gone (completely neutralized)?
We see bubbles when pouring the base over if the acid is still there. When we stop seeing bubbles, the acid is gone.
29
New cards
Is a neutralization reaction generally endothermic or exothermic?
Exothermic, as detectable heat is usually released.
30
New cards
What is a gas formation reaction?
A double replacement reaction where one of the products is not very stable and spontaneously decomposes to form water and a gas.
31
New cards
What are the two most common gas formation reactions?
* Carbonic acid, decomposing to form carbon dioxide gas and water
* Ammonium hydroxide, decomposing to form ammonia gas and water
* Ammonium hydroxide, decomposing to form ammonia gas and water
32
New cards
What is enthalpy?
Potential energy that may be gained or stored, usually lost as heat.
33
New cards
What is ΔH?
A change in energy
34
New cards
What is bond energy?
A measure of the bond strength of a chemical bond, and is the amount of energy needed to break the atoms involved in a molecular bond into free atoms. This is determined by the position of the negative electrons in an atom relative to the nucleus and the other nearby nuclei in a molecule.
35
New cards
What is bond energy a result of?
The chemical potential energy of intramolecular bonds.
36
New cards
What are intramolecular bonds?
The bonds between atoms in a molecule.
37
New cards
What are intermolecular bonds?
The bonds that exist between molecules.
38
New cards
What is the difference between the potential bond energy of reactants before and after a chemical or physical change called?
The enthalpy change, or ΔH value.
39
New cards
How does the energy change when breaking bonds differ from the energy change when forming bonds?
Bond breaking requires energy input while bond forming results in energy release.
40
New cards
What is the definition of an endothermic reaction?
If the energy necessary to initiate a chemical change (that is break the bonds in the reactants) is greater than the energy released when the bonds in the products form, the reaction is endothermic and requires a net input of energy (you spend more than you get).
41
New cards
What does an endothermic reaction entail in terms of energy?
* Net absorption of energy
* Gain in enthalpy
* Positive ΔH value
* Gain in enthalpy
* Positive ΔH value
42
New cards
What is the definition of an exothermic reaction?
If the energy necessary to initiate a chemical change (that is break the bonds in the reactants) is less than the energy released when the bonds in the products form, the reaction is exothermic and releases a net gain or energy (you get more than you spend).
43
New cards
What does an exothermic reaction entail in terms of energy?
* Net release of energy
* Decrease in enthalpy
* Negative ΔH value
* Decrease in enthalpy
* Negative ΔH value
44
New cards
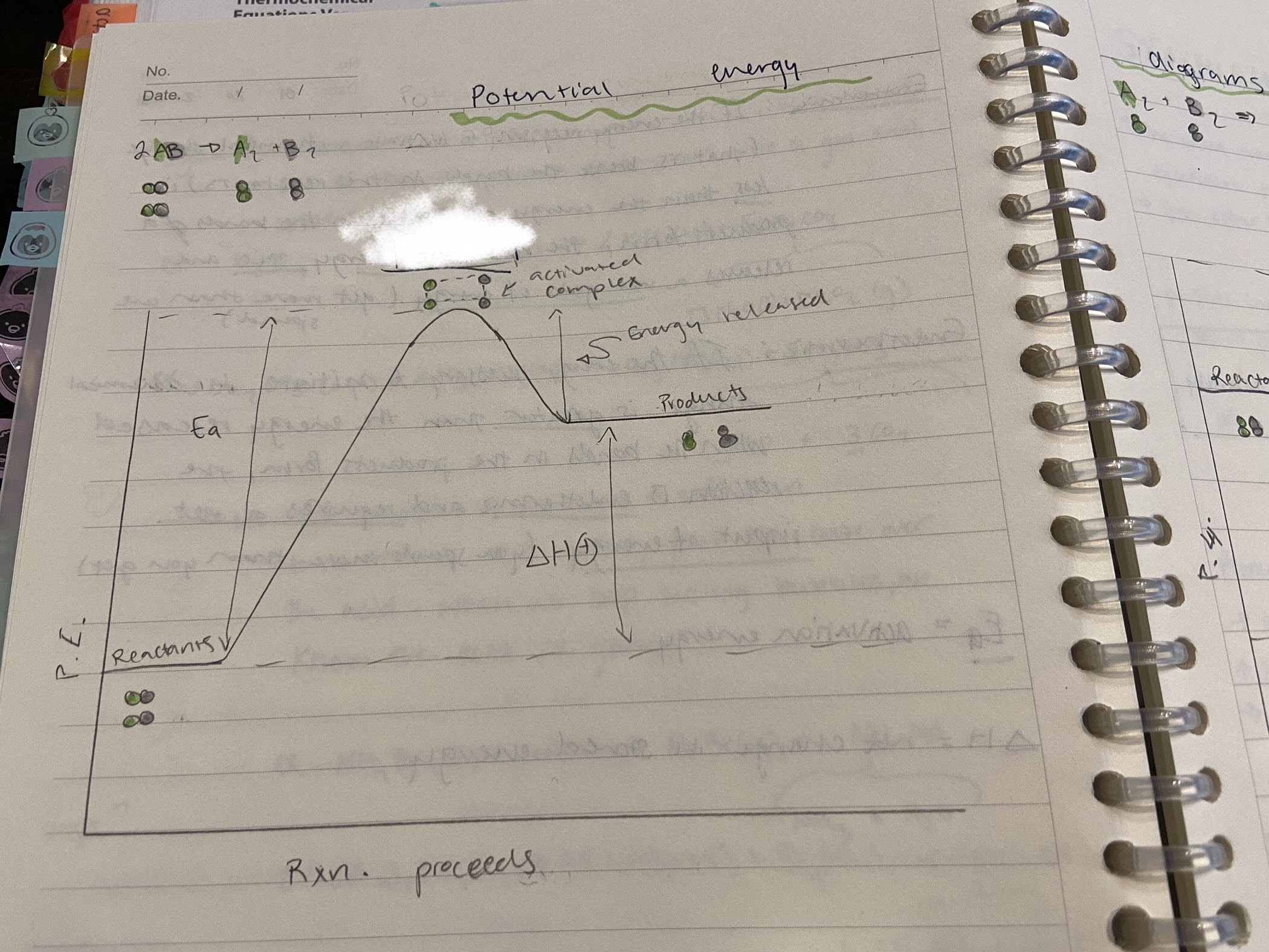
Is this reaction endothermic or exothermic?
Endothermic
45
New cards
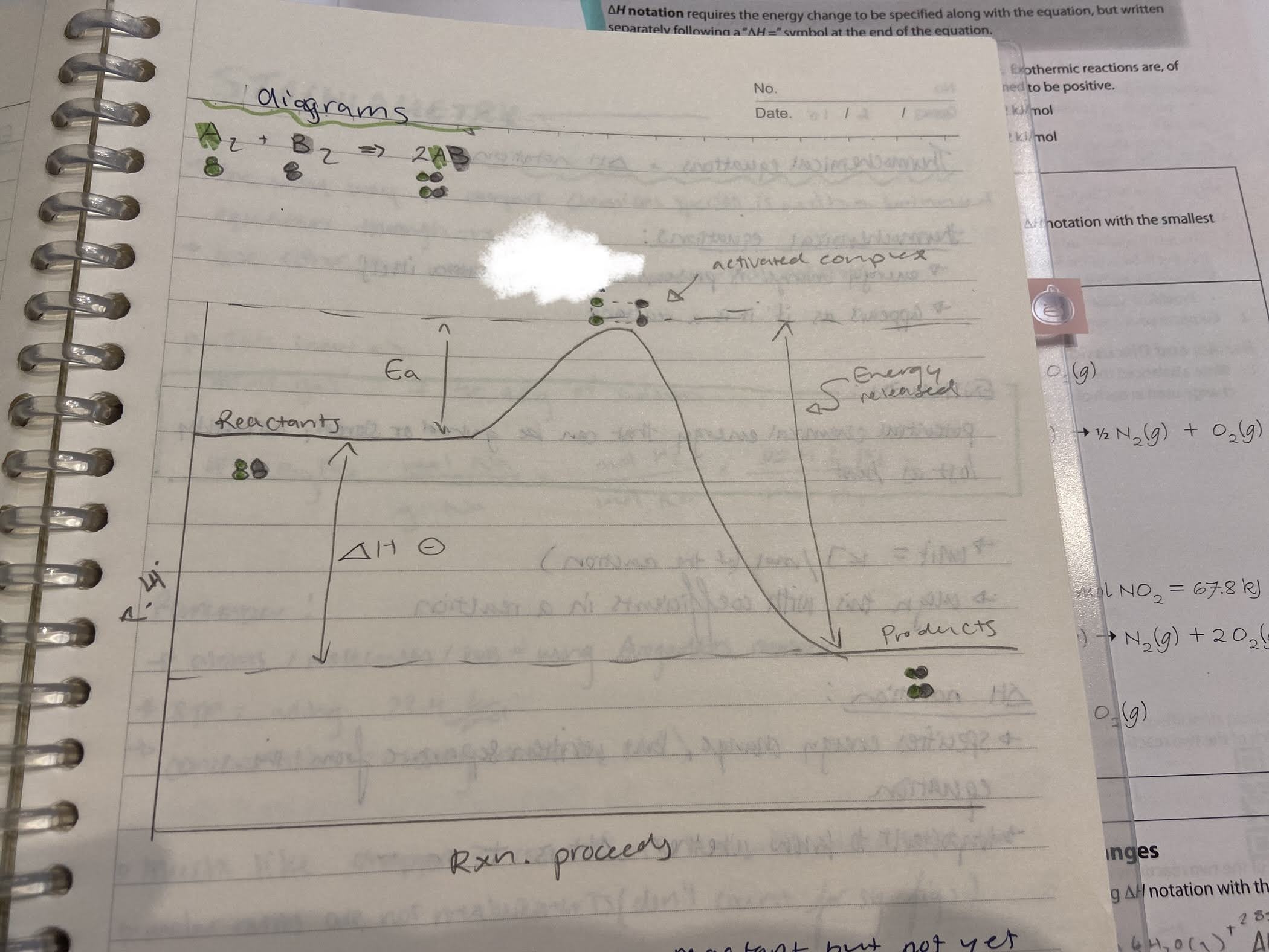
Is this reaction endothermic or exothermic?
Exothermic
46
New cards
How do we find the ΔH value?
P.E. of products - P.E. of reactants (P.E. can also be called enthalpy here)
47
New cards
What is the activated complex?
The state where the reactants are no longer reactants but not yet a product.
48
New cards
How do we find the activation energy?
P.E. of activated complex - P.E. of reactants?
49
New cards
How does energy in a compound correspond to attractive forces?
The less energy stored in a compound, the stronger the attractive forces. The more energy stored in a compound, the weaker the attractive forces. (Whenever attractive forces increase, energy is released)
50
New cards
Thermochemical equations vs. ΔH notation
Thermochemical equations:
* energy change is present in the equation itself as if it were a reactant itself
ΔH notation:
* specifies energy change, but written separate from the equation
* important to know whether it is a positive or negative value
* energy change is present in the equation itself as if it were a reactant itself
ΔH notation:
* specifies energy change, but written separate from the equation
* important to know whether it is a positive or negative value
51
New cards
Are energy (enthalpy) changes intensive or extensive properties?
Extensive properties.
52
New cards
What is important to remember when multiplying a reaction with energy notation involved?
When multiplying a chemical reaction to have whole number coefficients, whatever we multiply the equation by, we must also multiply the energy by.
53
New cards
What is stoichiometry used for?
It is used to study the relationships between the various elements and compounds involved in chemical reactions.
54
New cards
What does a balanced equation indicate?
It indicates the relative amounts of the chemicals involved in the reaction.
55
New cards
What do molar ratios tell us about the volume of gases?
Volumes of gases react in the same proportion as moles of gases. (mole ratios = volume ratios)
56
New cards
Why is mixing two solutions to react them so efficient?
The solvent pulls the ions apart. Mobile ions react faster with each other. Mixing two solutions is one of the fastest reactions we know of.
57
New cards
What is a limiting reactant or reagent?
The reactant that is totally consumed after a reaction. It limits the amount of product that can be formed.
58
New cards
What is the excess reactant or reagent?
The reactant that remains once the limiting reactant is completely consumed.
59
New cards
What are AMR and RMR?
AMR is the Actual Molar Ratio (what we currently have). RMR is the Required Molar Ratio (as per the balanced chemical equation).
60
New cards
What is the percentage purity of a chemical?
(mass of pure chemical/mass of impure sample) x 100%
61
New cards
Why is percentage purity important?
The only thing that goes in and out of a stoichiometric reaction is pure, so we need to know how much of a chemical we really have.
62
New cards
What yield does stoichiometry give us and why is it inaccurate?
Stoichiometry gives us the theoretical yield. It is inaccurate as most reactions complete themselves only partially, resulting instead in a percentage yield.
63
New cards
What is the percentage yield?
(Actual Yield/Theoretical Yield) x 100%