Factors that affect equlibrium
1/5
Earn XP
Description and Tags
- explain the effect of changes of concentration and pressure on chemical systems at equilibrium by applying collision theory to the forward and reverse reactions - explain and predict the effect of temperature change on chemical systems at equilibrium by considering the enthalpy change for the forward and reverse reactions - apply Le Châtelier’s principle to predict the effect changes of temperature, concentration of chemicals, pressure and the addition of a catalyst have on the position of equilibrium and on the value of the equilibrium constant
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
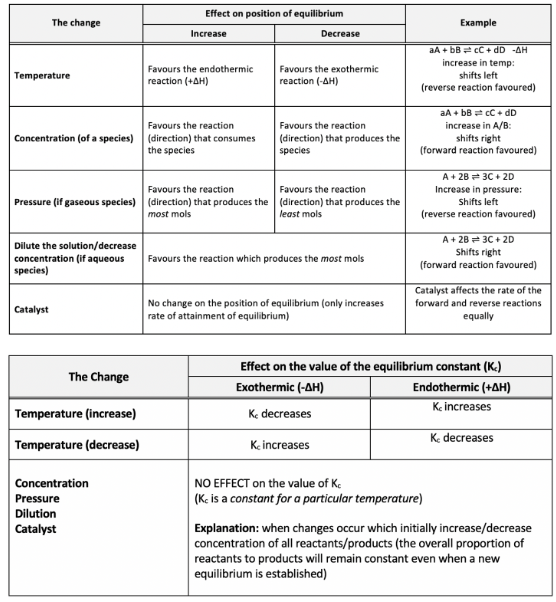
Increased temperature
Greater proportion of particles have sufficient energy to overcome the activation energy
and since the $E_a$(endothermic reaction) = $E_a$(exothermic reaction) + $|ΔH|$) the position of equilibrium will shift to favour the endothermic reaction.
Decreased temperature
lower proportion of particles have sufficient energy to overcome activation energy
therefore the position of equilibrium will shift to favour the exothermic reaction
increased pressure/concentration
causes higher collision frequency → thus increasing the amount of successful collisions between particles
in an equilibrium system this increases the rate of the reaction which has more mols of reactants
favouring the reaction (direction) which produces the least mols
decreased pressure/concentration
causes a lower collision frequency, thus decreasing the amount of successful collisions between particles
in an equilibrium system this decreases the rate of the reaction which has more mols of reactant
favouring the reaction (direction) which produces the most mols
Le Châtelier’s principle
states that if a stress is applied to a system in equilibrium, the system will shift (partially adjust) in order to minimise the effect of the stress
Le Châtelier’s principle: changes of temp, concentration, pressure and the addition of a catalyst