Electron Pair Geometries + Molecular Geometry + Hybridization Diagram | Quizlet
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
Linear
2 pairs
No lone pairs = Linear (180°)
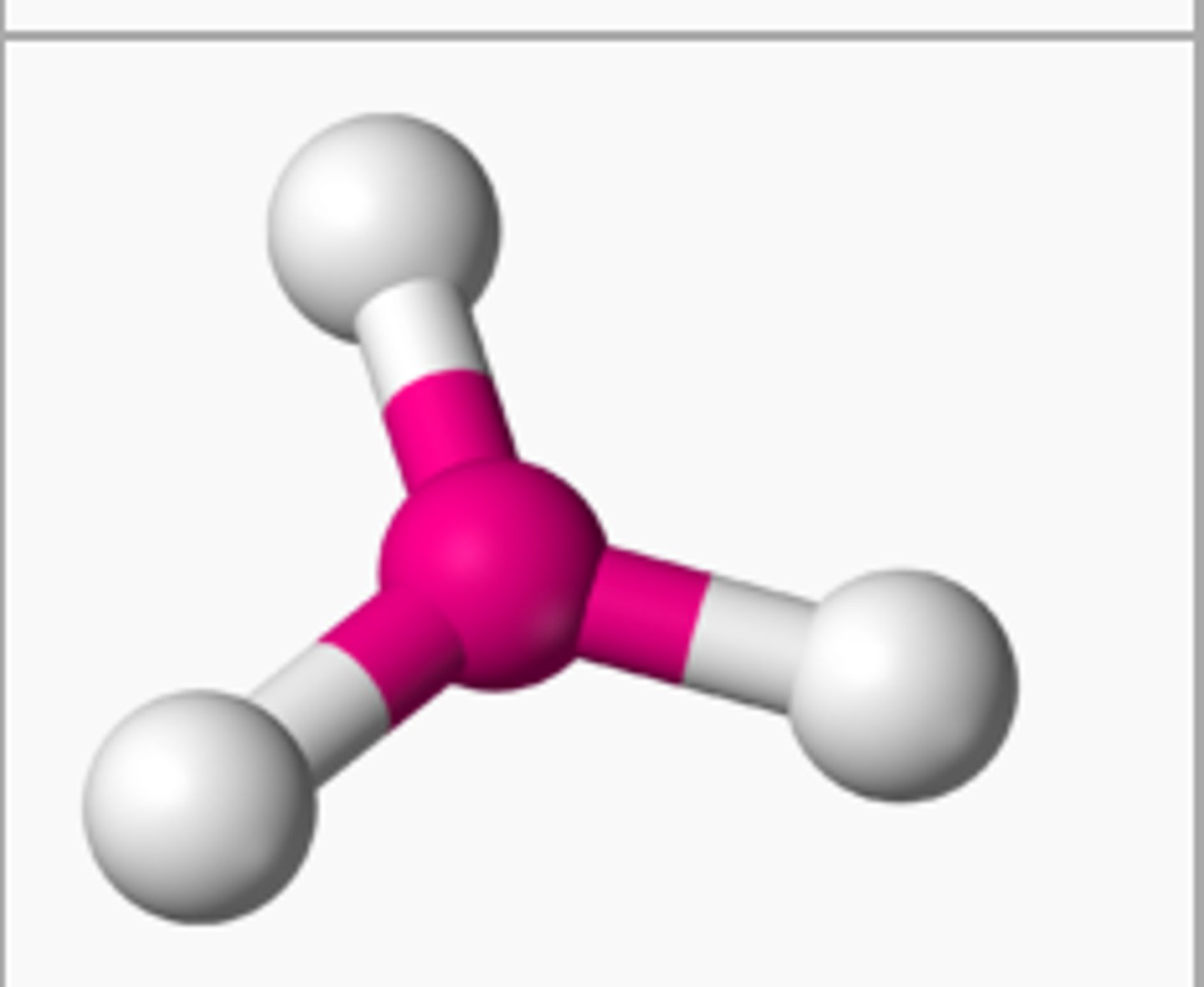
Trigonal Planar
3 Electron Pairs
-
0 lone pairs = Trigonal Planar (120°)
1 lone pairs = Bent (<120°)
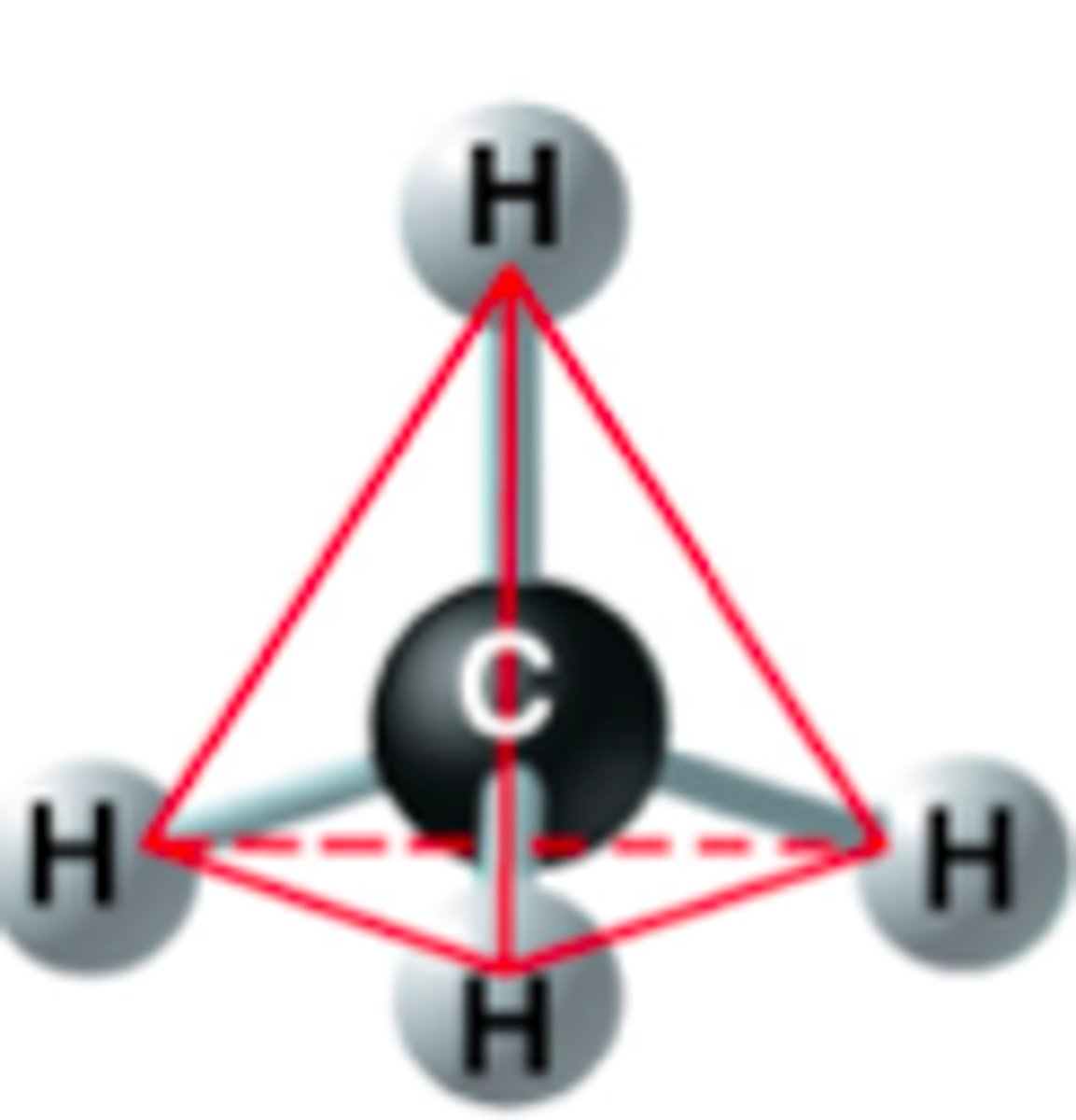
Tetrahedral
4 Electron Pairs
-
0 lone pairs = Tetrahedral (109.5°)
1 lone pairs = Trigonal Pyramid (<109.5° ~107°)
2 lone pairs = Bent (<109.5° ~105°)
Trigonal Bipyramidal
5 Electron Pairs
-
0 lone pairs = Trigonal Bipyramidal (90°, 120°)
1 lone pairs = Seesaw (<90°, <120°)
2 lone pairs = T structure (<90°)
3 lone pairs = Linear (180°)
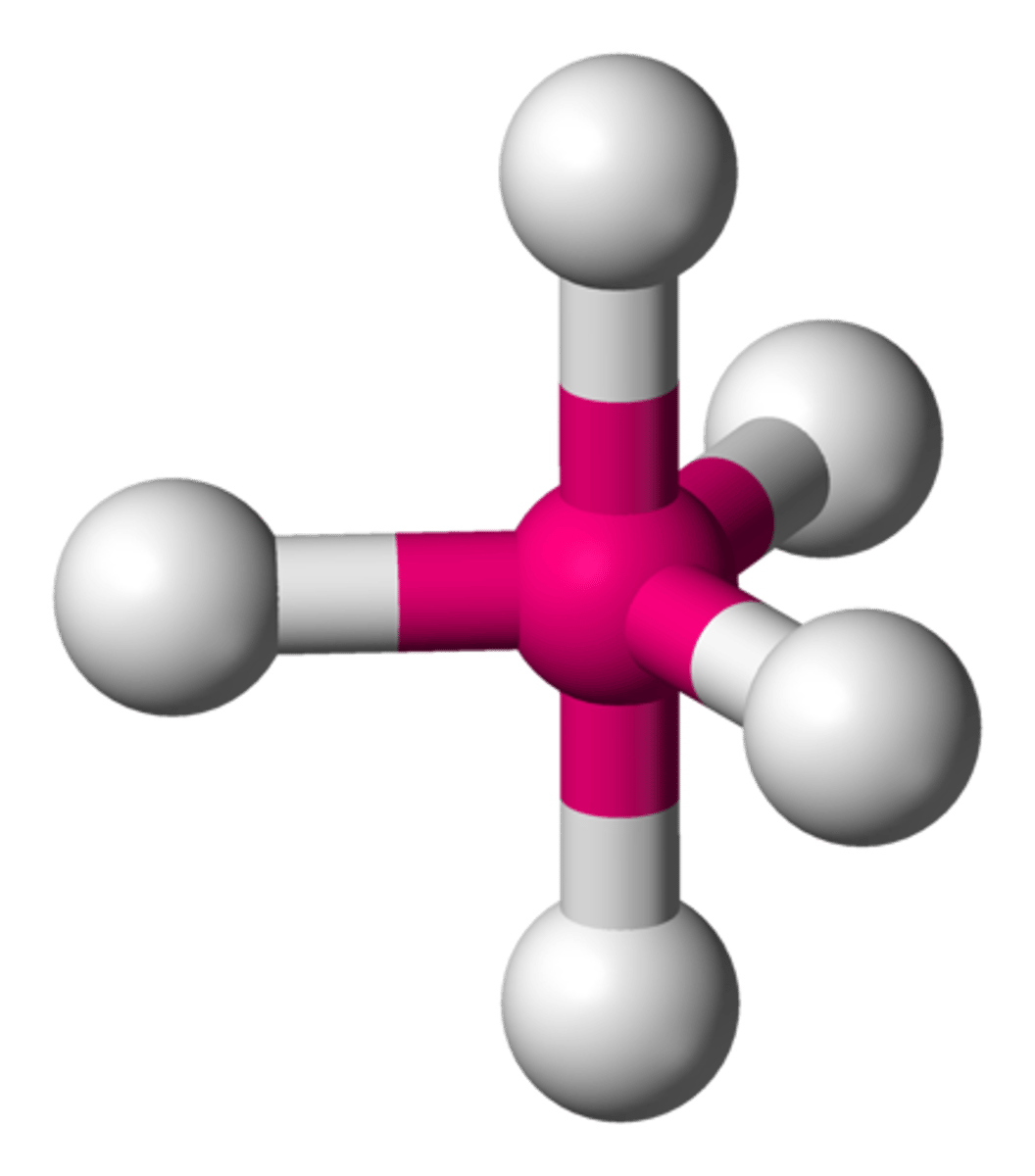
Octahedral
6 Electron Pairs
-
0 lone pairs = Octahedral (90°, 90°)
1 lone pairs = Square Pyramidal (90°, <90°)
2 lone pairs = Square Planar (90°)
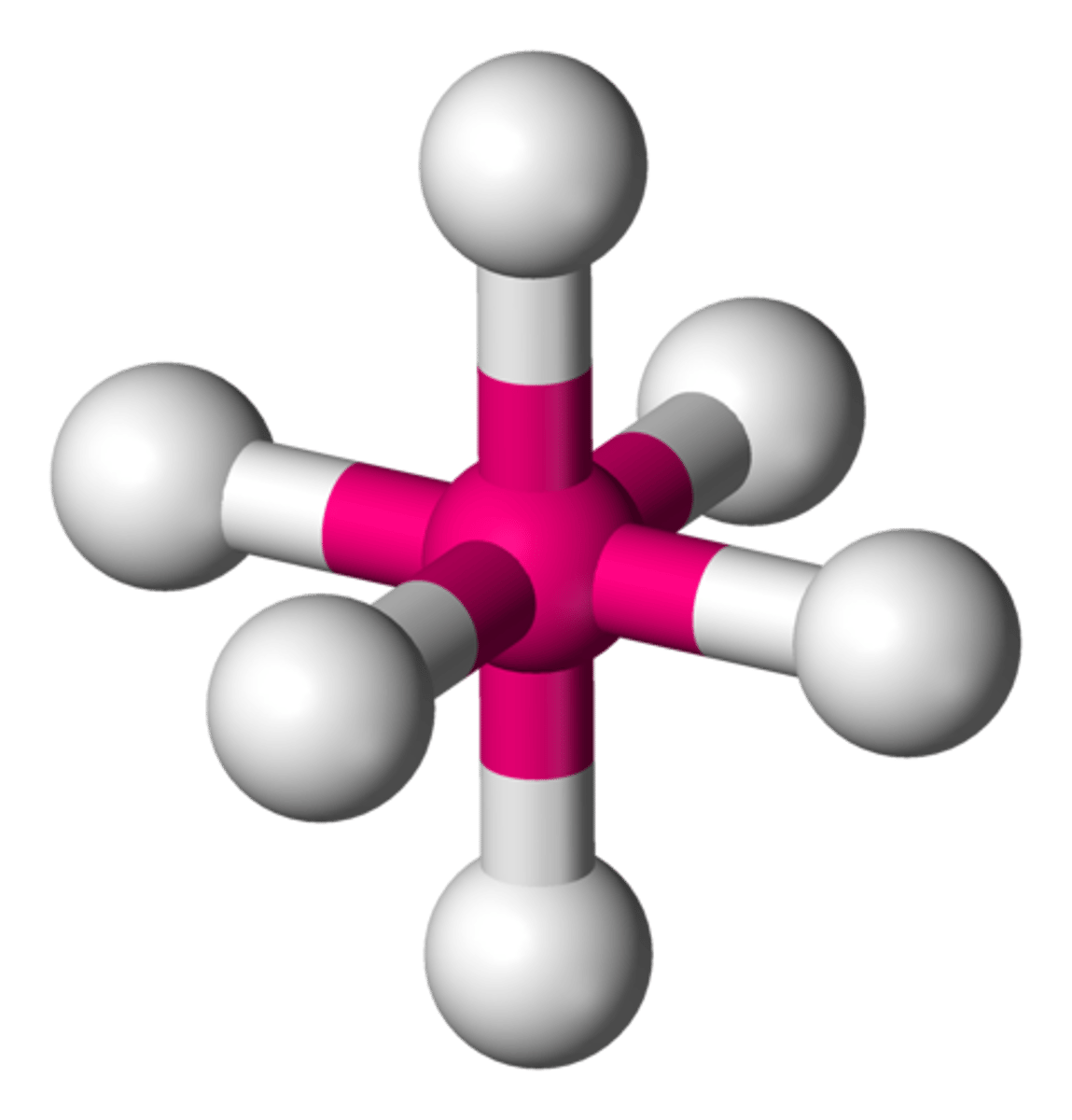
TERM
Electron Geometry
DEFINITION
describes the structure of the electron pairs around the central atom
TERM
Molecular Shape
DEFINITION
The shape (also called molecular geometry) is the arrangement of atoms around the central atom.
*The geometry is defined by the atoms NOT the electron pairs.
VESPR Theory (Valence Shell Electron Pair Repulsion)
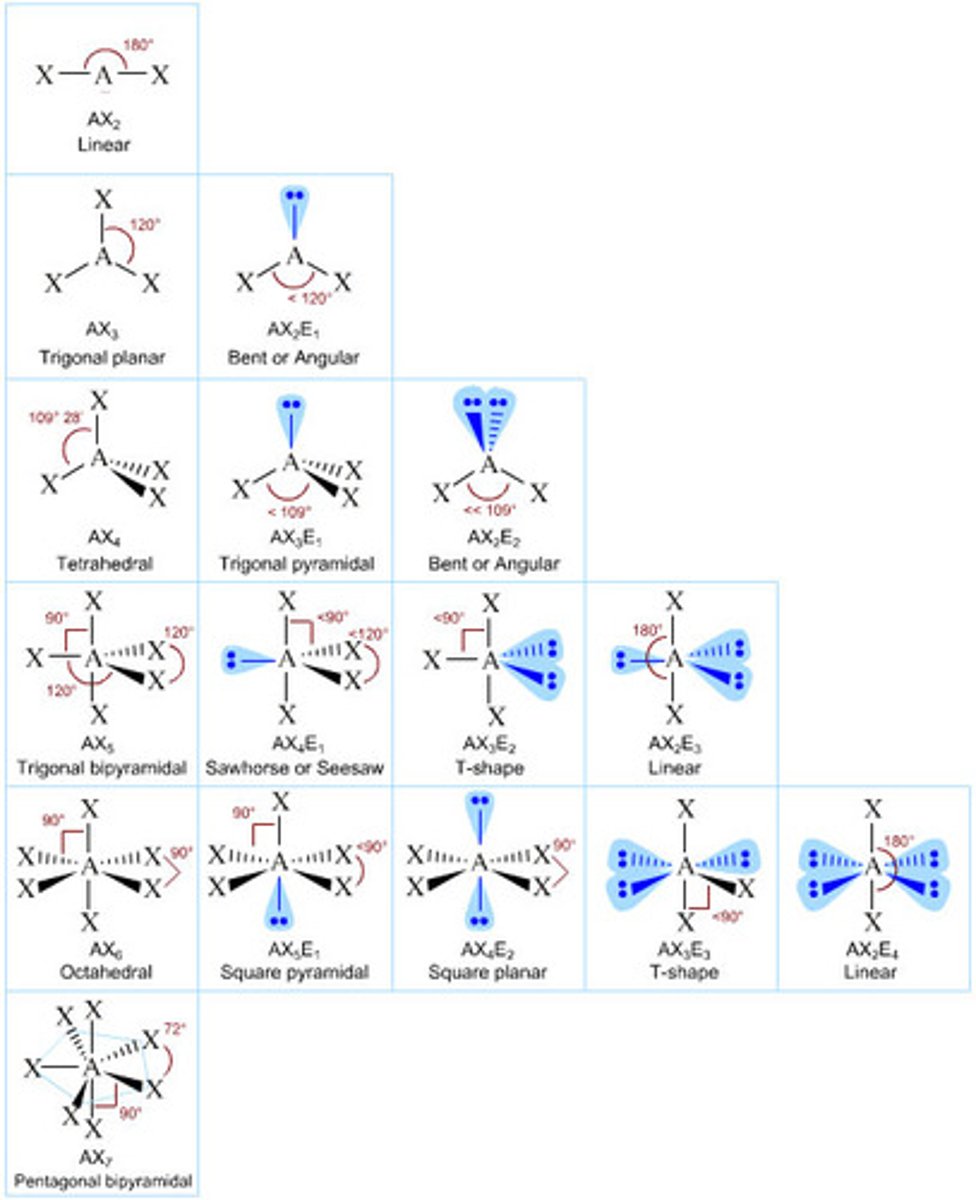
electron geometry catagories
Linear, Trigonal Planar, Tetrahedron, Trigonal Bipyramidal, Octahedral
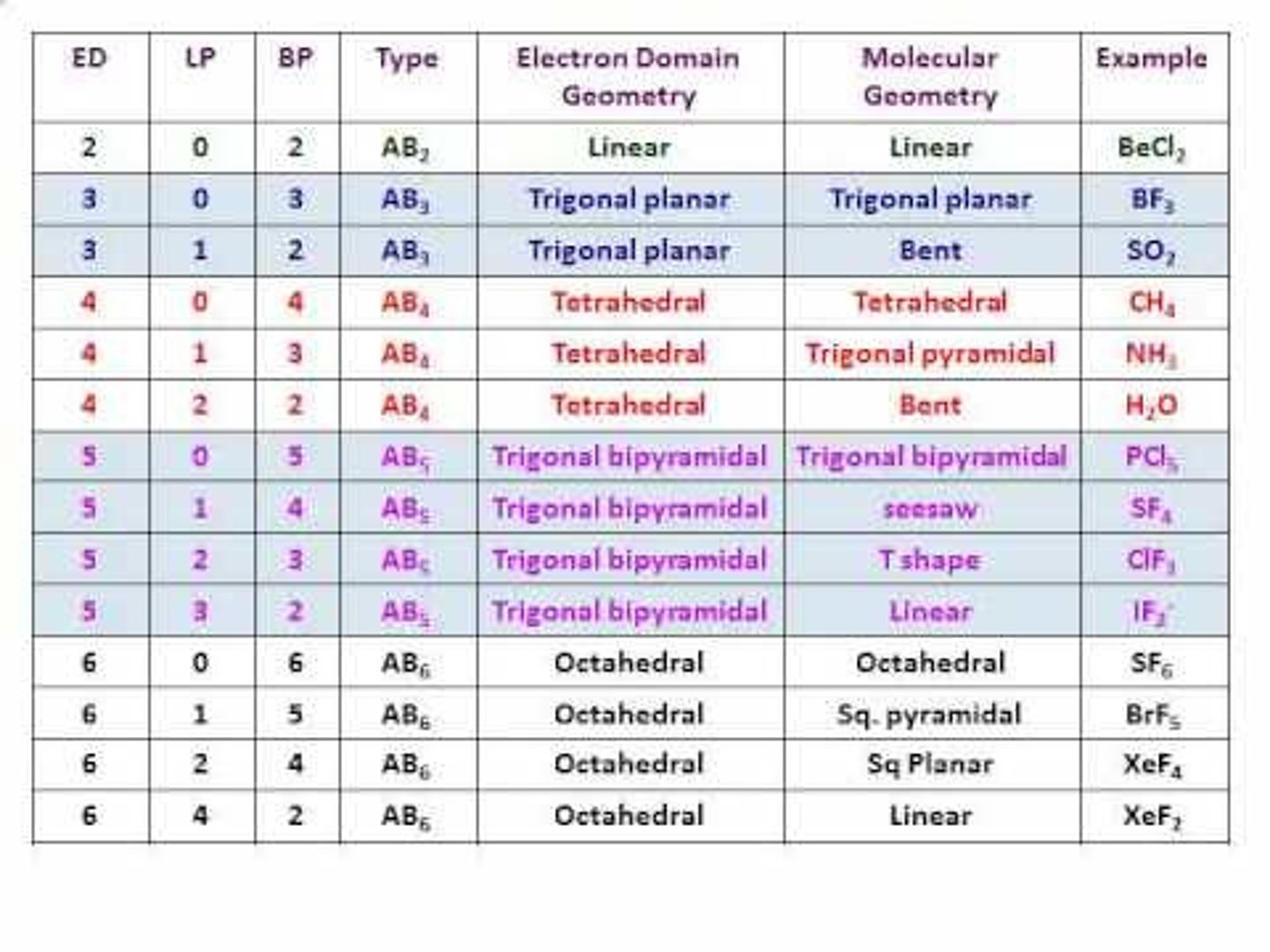
Molecular shape is the same as the electron pair geometry when
none of the structural electron pairs are non bonding (lone pair) electrons.
no lone pairs on central atom
The shape is the same as the electronic geometry.
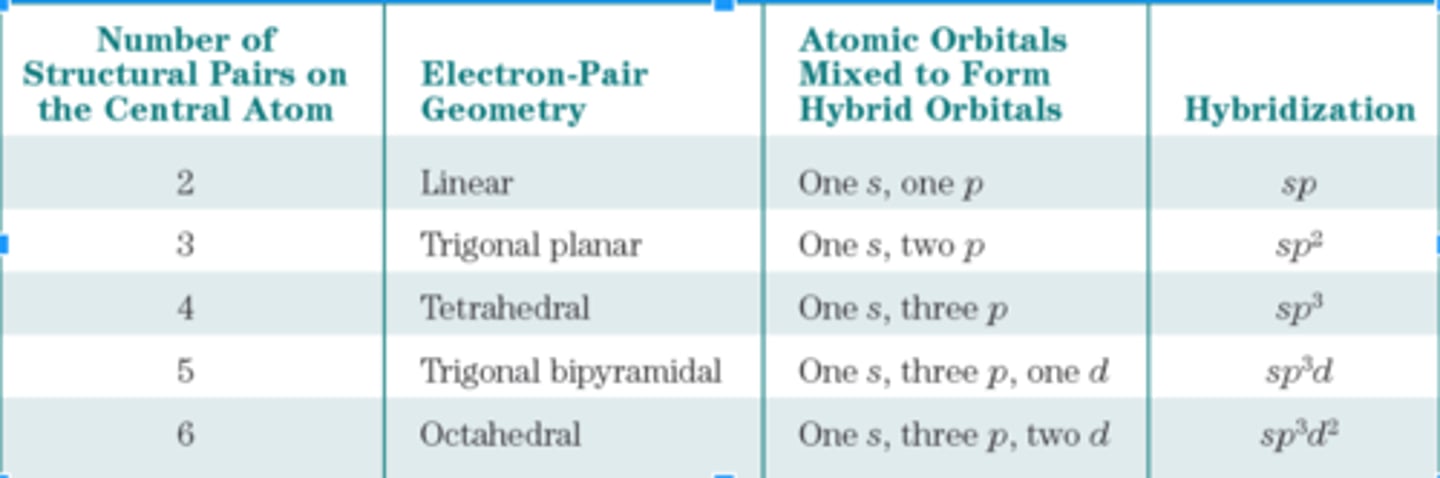
hybrid orbitals
orbitals of equal energy produced by the combination of 2 or more orbitals on the same atom
Relation between Structural Pairs and Hybridization
Are lone pairs included in determining the electron pair structure?
yes
The number of structural pairs is equal to
the number of atoms bonded to a central atom + the number of lone pairs on the central atom.
if molecular shape and electron pair geometry are the same and the surrounding atoms are the same , then
the molecule is non-polar exception is square planar
3 part valence bond theory:
1. Valence atomic orbitals on adjacent atoms overlap.
2. Each pair of overlapping valence orbitals is occupied by two valence electrons to form a chemical bond.
3. Valence electrons are either involved in bonding between two atoms (shared bonding pairs) or reside on a single atom (nonbonding lone pairs).
A px orbital can form a σ bond with
another px orbital, an s orbital, or a dx2-y2 orbital. The other two orbitals, py and dxy, do not have lobes of electron density that point along the x- axis.
hybrid orbitals
equal-energy orbitals that are the combination of an atom's atomic orbitals.
Relation Between Structural Pairs and sp, sp 2, and sp 3 Hybridization
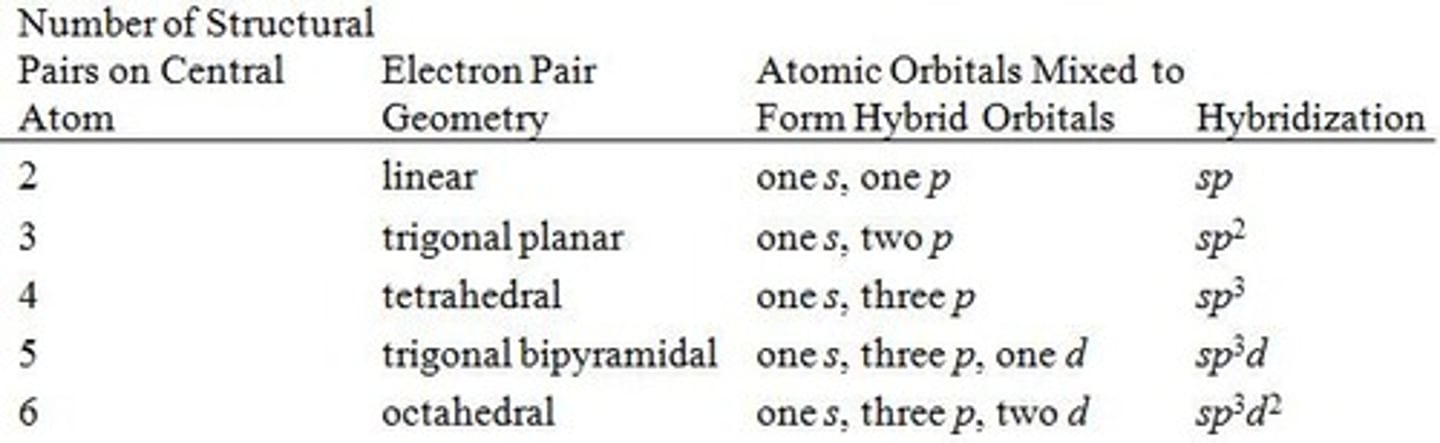
A single bond (one line) represents
a sigma bond
a double bond (two lines) represents
one sigma bond and one pi bond
a triple bond (three lines) represents
one sigma bond and two pi bonds.
the pi bond is a weaker bond than the sigma bond because
pi bond is sideways overlap, sigma is direct head on overlap of orbitals
Relationship between Hybridization and Number of Possible Pi Bonds.
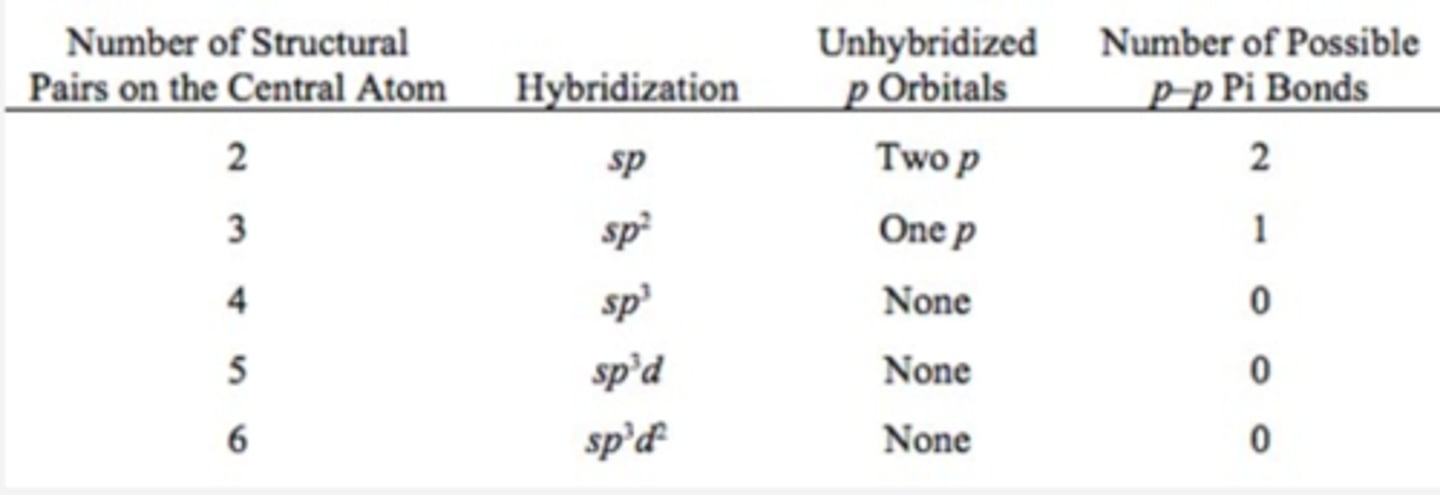
Can compounds with 5 or 6 structural pairs form p-p pi bonds?
NO
Isomers
two or more substances that have the same chemical formula but have different properties because of the different arrangement of atoms. Molecules with pi bonds are one example of compounds that can exist as more than one isomer.
p orbitals at 90 degrees
do not form pi bonds
sigma bonds are
hybridized
pi bonds are
unhybridized
molecular orbital (MO) theory
Developed by Robert Mullikan
Orbitals in a molecule are delocalized (they are shared in the molecule) and treated as waves.
Predicts the shapes and energies of orbitals that contain no electrons.
Predicts paramagnetism much more accurately
Orbitals in the MO theory
Bonding orbitals in sigma and pi
Increase electron density between atoms
predicts bonding
Antibonding sigma or pi
describes interference between electron waves.
no electrons between atoms = no bonding
No bonding orbitals.
molecular geometry
is defined by the atoms NOT the electron pairs.
anti-bonding orbitals are ________ in energy
higher
Drawing Molecular orbital diagrams
1.Identify valence orbitals
2.Place the valence on the outside of the drawing
molecular orbitals are placed between with one bonding and one antibonding for each valence orbital
3.Then connect the valence orbitals to the molecular orbital via a dashed/dotted line to 4.show how we are forming the orbitals.