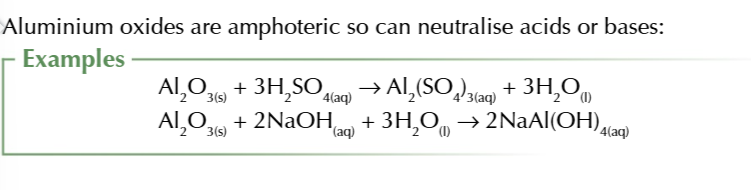PERIOD 3 ELEMENTSSS
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
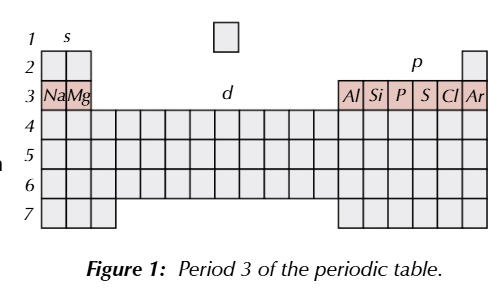
3rd row is periodic table
sodium
magnesium
aluminium
silicon
phosphorus
sulfur
chlorine
argon
sodium is more reactive w water than magnesium
because it takes less energy to lose one electron that lose 2 so more energy is needed for magnesium to react
sodium react vigorously w cold water forming a molten ball on the surface, fizzing and producing H2 gas
the reaction produces sodium hydroxide so forms a strong alkaline solution (pH 12-14)
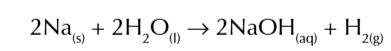
magnesium reacts very slowly with cold water and forms a weak alkaline solution
(pH 9-10)
the solution is only weakly alkaline because magnesium hydroxide is not very soluble in water so a few OH- ions are produced
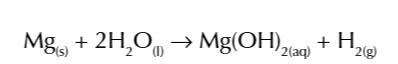
magnesium reacts must faster w steam
forming magnesium oxide
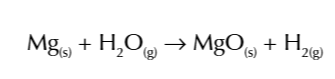
period 3 elements react w oxygen to form compounds where they are in theri higest oxidative state, corresponding to their group number
example phosphorus ion is +5
Sulfur is a exception
SO2: formed at normally +4
SO3: formed at high temp and a catalyst +6
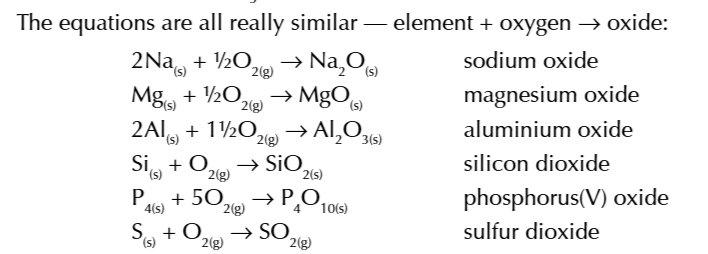
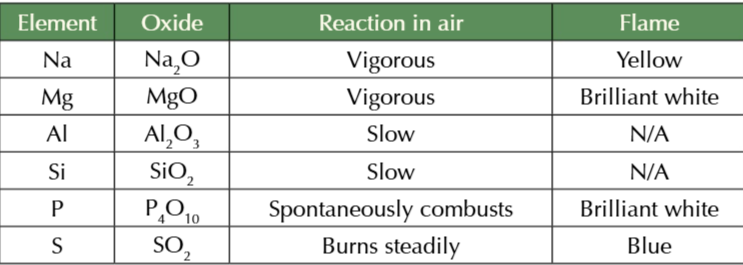
period 3 metal oxide melting points
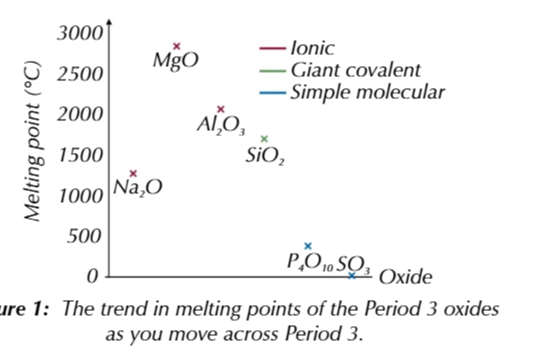
Na2O, MgO and Al2O3 are all metal oxides w high melting points
because the form giant ionic lattices
Na2O melting point <MgO melting point
because Mg2+ attract O2- stronger than Na+ ions
MgO melting point > Al2O3 melting point
because there is only a small difference between Al’s electronegativity and Oxygens compared to between magnesium and oxygen (larger difference in electronegativity)
because of the small electronegativity between Al2O3 molecules
O2- in the compound cant attract electrons in the metal oxygen bond as strongly as MgO making Al2O3 partially covalent
SiO2 has a higher melting point that other non metal oxides because it has a giant macromolecular structure
strong covalent bonds sold the structure so lots of energy is required to break the bonds giving a high melting point temp
P4O10 and SO3 are covalent molecules with simple molecular structures
molecules are held together by weak intermolecular forces which take little energy to overcome
NaOH is more water soluble than Mg(OH)2
so Na + water produces a more alkaline solution that Mg + water as more OH- are released w NaOH
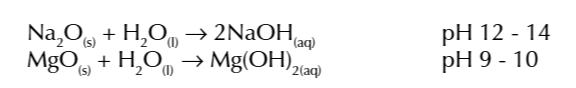
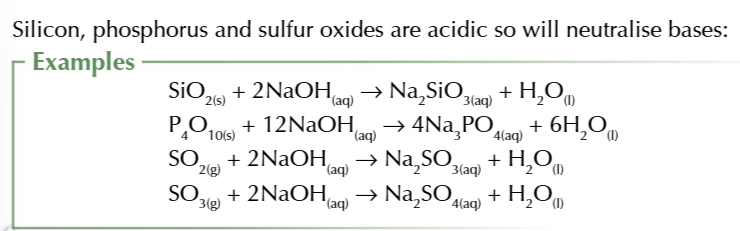
simple covalent oxides of non-metals like phosphorus and sulfur form acidic solutions
all of the acids are strong w very low pH 0-1 for solutuons w a conc of atleast 1 mol dm-3
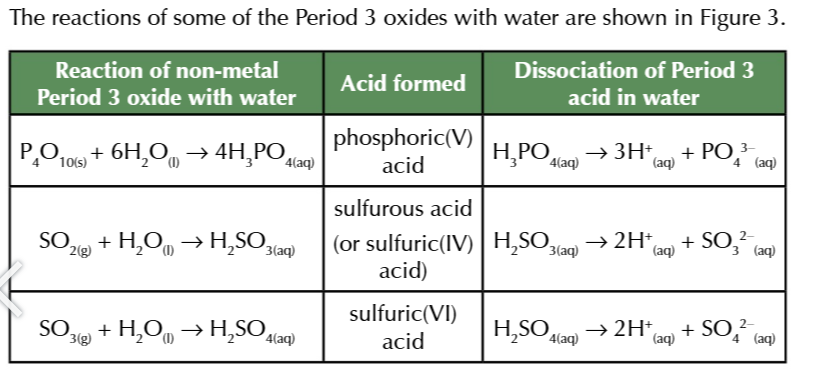
silicon dioxide has a giant covalent structure that is insoluble in water
silicon dioxide DOES however react w bases to form salts so its cassified as acidic
aluminium oxide is partially ionic and partially covalent is also insoluble in water
aluminium oxide reacts w acids and bases to form salts to aluminium oxide is amphoteric
Base + acid → salt + water
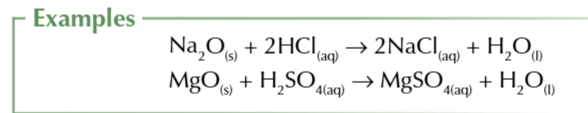
acid + base → salt + water
aluminium’s trifflin ahh