INSTRUMENTAL METHODS OF ANALYSIS (SPECTROSCOPY ONLY)
1/91
Earn XP
Description and Tags
mag ppt/trans nalang sa crude drug and chromatography tamad na ko
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
92 Terms
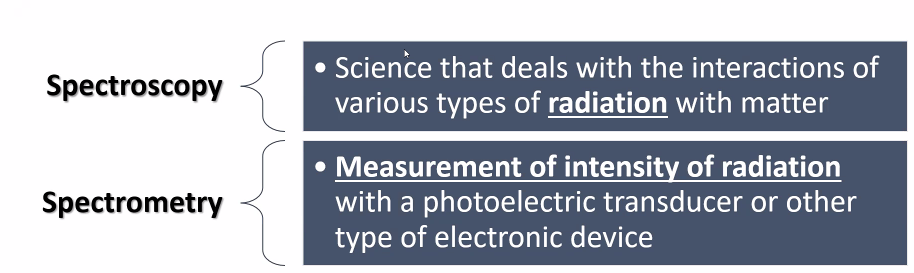
Spectroscopy
Science that deals with the interactions of various types of radiation with matter
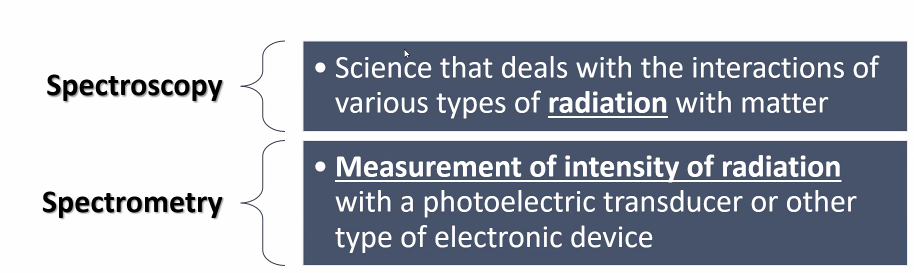
Spectrometry
Measurement of intensity of radiation with a photoelectric transducer or other type of electronic device

Electromagnetic radiation
Type of radiation that has both magnetic and electric fields, with characteristics of both particle and wave.
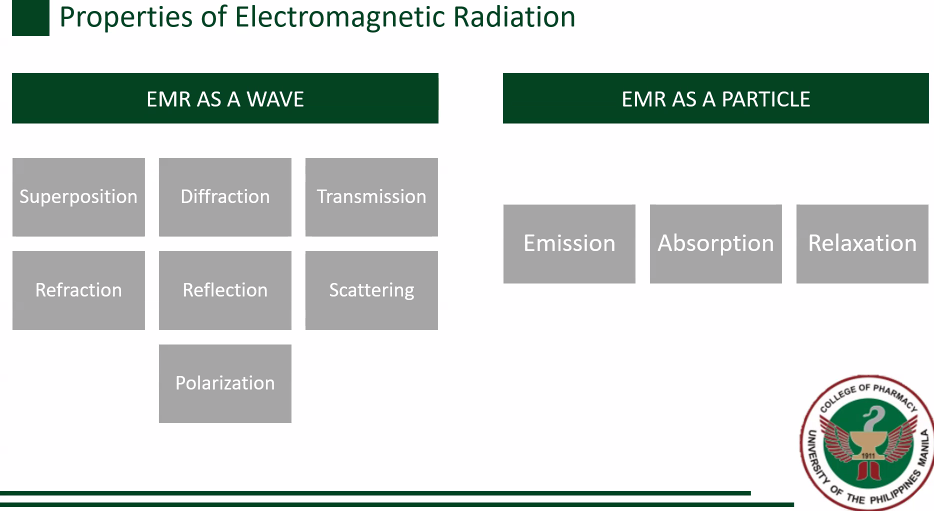
Superposition
Diffraction
Transmission
Refraction
Reflection
Scattering
Polarization
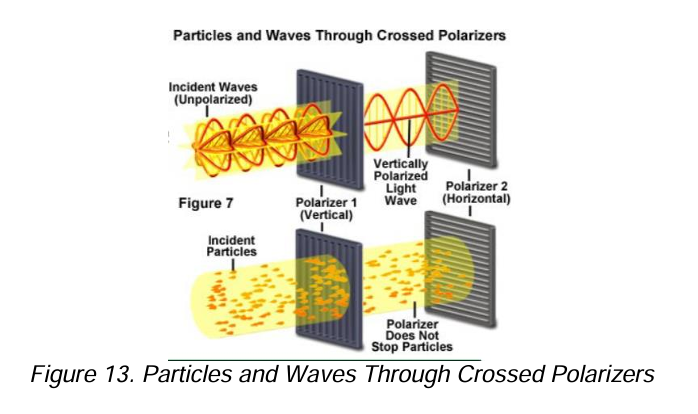
EMR as a wave [7]
Emission
Absorption
Relaxation
EMR as a particle [3]
Superposition
[EMR as a wave]
When two or more waves overlap in space, the resulting disturbance is equal to the algebraic sum of the individual disturbances
Diffraction
[EMR as a wave]
The bending and spreading of waves around an obstacle
Transmission
[EMR as a wave]
The passage of electromagnetic radiation through a medium.
Refraction
[EMR as a wave]
When light waves change direction as they pass from one medium to another
Reflection
[EMR as a wave]
When incident light (incoming light) hits an object and bounces off.
Scattering
[EMR as a wave]
When light bounces off an object in a variety of directions. The amount depends on the wavelength of the light and the size and structure of the object
Polarization
[EMR as a wave]
Describes the magnitude and the direction of the electric field of the wave.
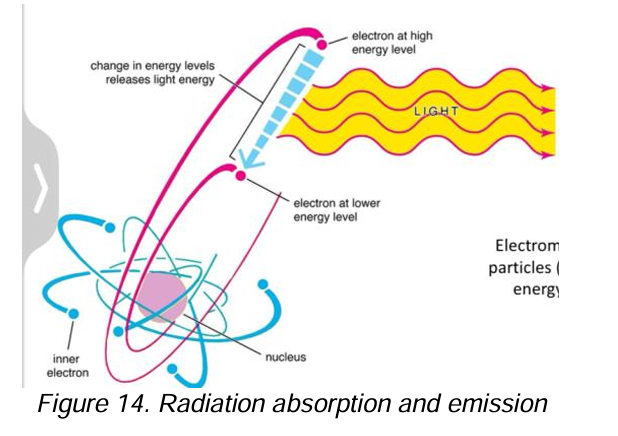
Emission
[EMR as a particle]
When a substance gives off electromagnetic radiation
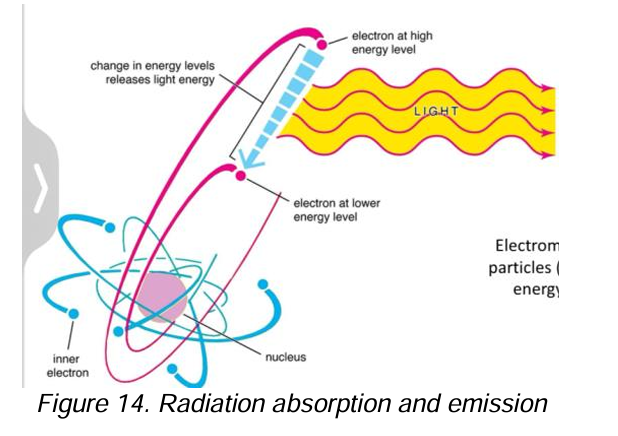
Absorption
[EMR as a particle]
When electrons in a substance take up energy from electromagnetic radiation types of light
Relaxation
[EMR as a particle]
When electrons fall from an excited state to a lower energy state (usually the ground state).
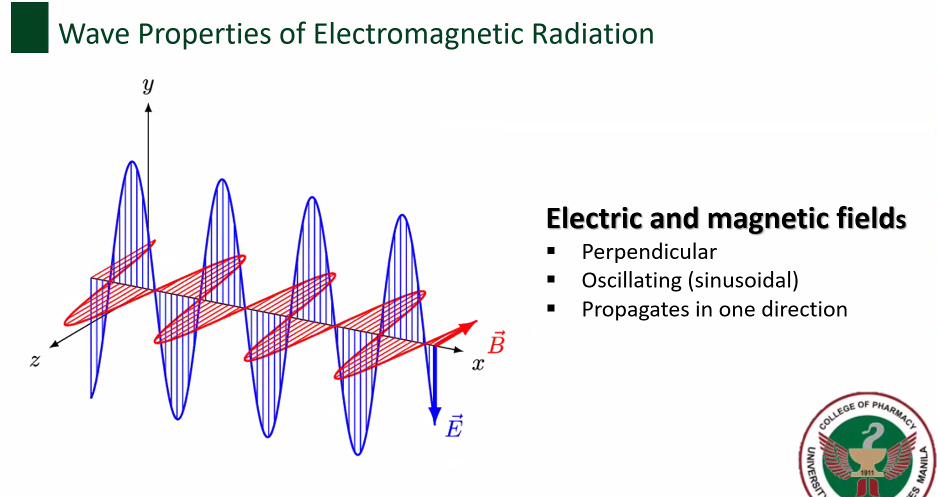
Electric and magnetic fields
Amplitude
Wavelength (λ)
Frequency (v)
Wave properties of electromagnetic radiation [4]
Perpendicular
Oscillating (sinusoidal)
Propagates in one direction
Electric and magnetic fields [3]
Amplitude
[Wave property of electromagnetic radiation]
Length at a maximum in a wave
Wavelength (λ)
[Wave property of electromagnetic radiation]
Crest-to-crest distance between waves
Frequency (v)
[Wave property of electromagnetic radiation]
Number of complete oscillations per second
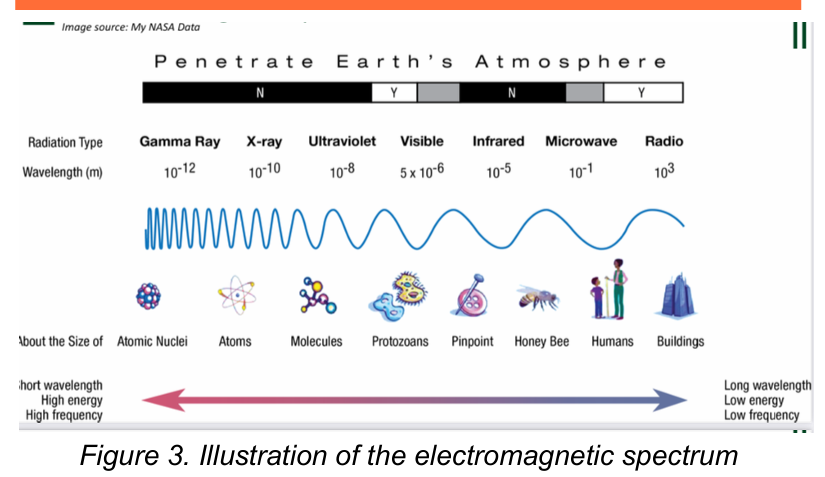
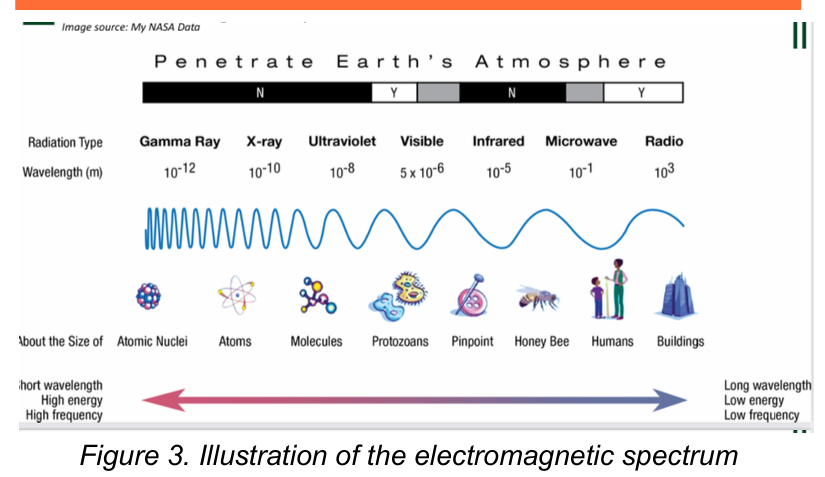
Electromagnetic Spectrum
Electromagnetic Spectrum
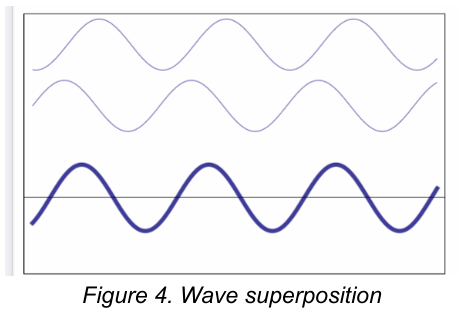
Superposition of waves
States that when two or more waves traverse the same space, a disturbance occurs that is the sum of the disturbances caused by the individual waves
Constructive interference
Destructive interference
Beats
Superposition of waves [3]
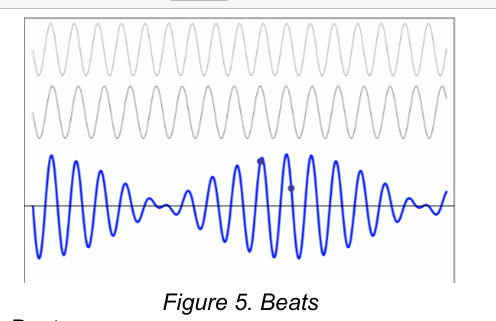
Beats
When two waves have identical amplitude but different frequencies, the resulting wave is no longer sinusoidal. It exhibits a periodicity or beat.
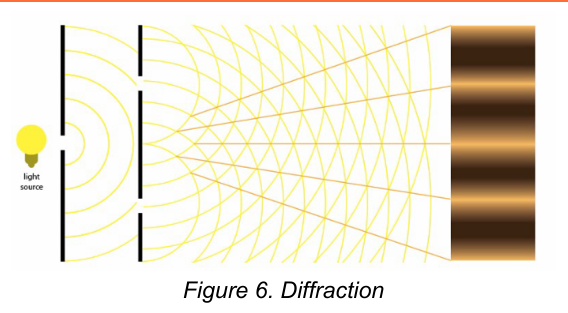
Diffraction
is a process in which a parallel beam of radiation is bent as it passes by a sharp barrier or through a narrow opening.
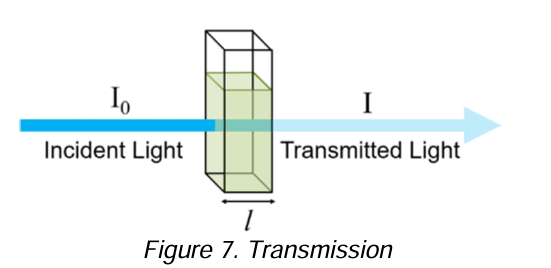
Transmission
is the propagation of radiation through a medium and depends upon the kinds and concentrations of atoms, ions, or molecules in the medium
Refraction
When radiation passes at an angle through the interface between two transparent media that have different densities, an abrupt change in direction, of the beam is observed because of a difference in velocity of the radiation in the two media.
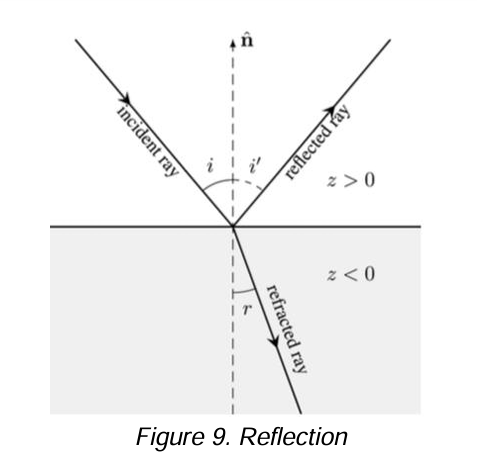
Reflection
When radiation crosses an interface between media that differ in refractive index, this occurs
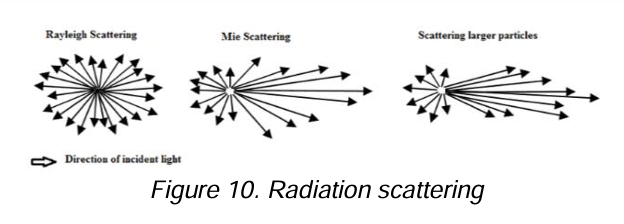
Scattering
A very small fraction of the radiation is transmitted at all angles from the original path and that the intensity of its scattered radiation increases with particle size.
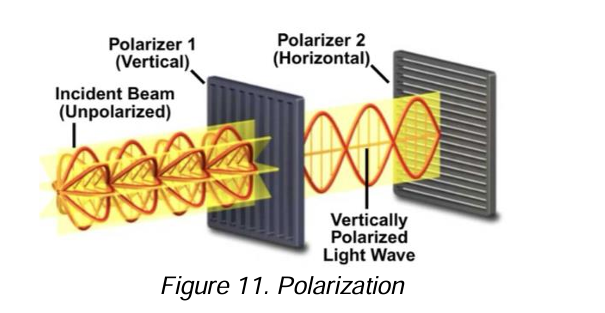
Polarization
Polarized ultraviolet and visible radiation is produced by passage of radiation through media that selectively absorb, reflect, or refract radiation that vibrates in only one plane.
Quantum Theory
Atoms, ions, and molecules can exist only in certain discrete states, characterized by definite amount of energy. When a species changes its state, it absorbs or emits an amount of energy exactly equal to the energy difference between the states.
Quantum Theory
When atoms, ions, or molecules absorb or emit radiation in making the transition from one energy state to a second, the frequency or the wavelength of radiation is related to the energy difference between the states by the equation: *see pic
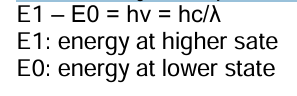
Wavelength range: 180-750 nm
Quantum transition: bonding electrons
Wavelength range and quantum transition of UV/Vis absorption, emission and fluorescence
Wavelength range: 0.78-300 μm
Quantum transition: Rotation/vibration of molecules
Wavelength range and quantum transition of IR absorption and Raman scattering
Excitation of the electrons
Radiation in the UV/Visible region is absorbed through [BLANK] involved in the bonds between the atoms making up the molecule so that the electron cloud holding the atoms together redistributes itself and the orbitals occupied by the bonding electrons no longer overlap.
A. The first statement is true. The second statement is false.
Chromophore = if there are double/triple bonds in the compound
I. No chromophore, nothing to be determined in the sample or standard
II. It can still be considered a chromophore when there are only single bonds present in a compound.
A. The first statement is true. The second statement is false.
B. The first statement is false. The second statement is true.
C. Both statements are true.
D. Both statements are false.
True.
It means that is is capable of absorbing light that can elicit a value of Absorbance in the spectrophotometer.
[T/F] For a compound to be suitable for UV/Vis spectra, it should contain a chromophore
False.
[T/F] Gamma rays have the highest wavelengths and shortest energy in the visible region.
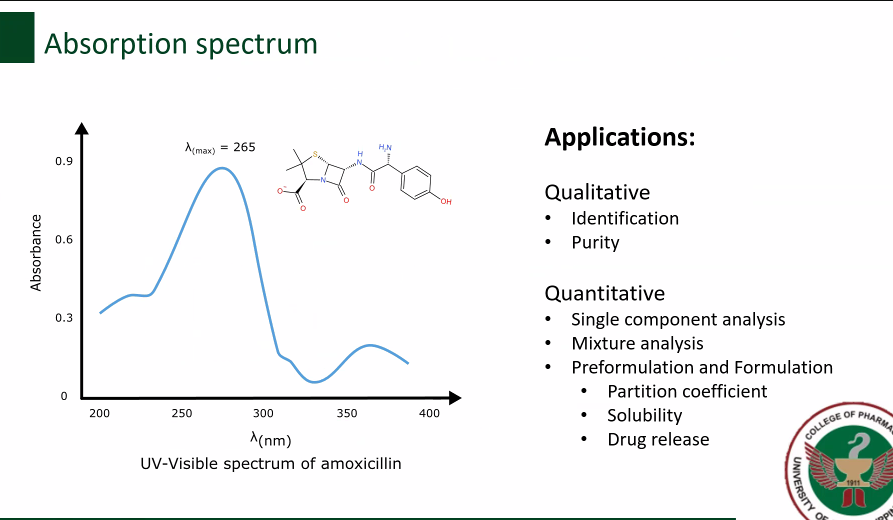
Identification
Purity
Applications of Absorption spectrum (Qualitative) [2]
Single component analysis
Mixture analysis
Preformulation and Formulation
Partition coefficient
Solubility
Drug release
Applications of Absorption spectrum (Quantitative)
Where we can get the most accurate results
Why measure at lambda max?
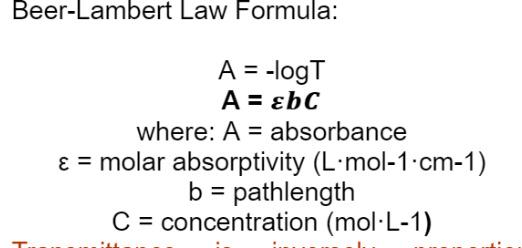
Beer-Lambert Law Formula
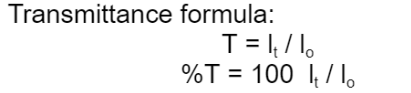
inversely
Transmittance is [directly/inversely] proportional with Absorbance.
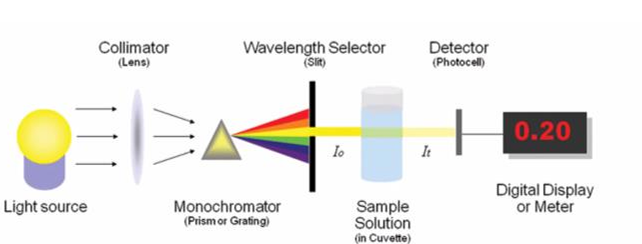
Light source → Collimator (lens) → Monochromator (Prism or Grating) → Wavelength Selector (Slit) → Sample solution (in cuvette) → Detector (Photocell) → Digital Display or Meter
How a Spectrophotometer works
Conjugation
Presence of Auxochrome
Polarity of Solvent
Absorption shifts properties [3]
![<p><strong>Absorption shifts properties</strong><span style="color: red"><strong> [3]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/31cc0b81-c2f2-4730-817f-282534a4ac41.png)
increases
During Conjugation, as the number of pi electrons increases, the localization [increases/decreases]
Auxochrome
Functional group that does not itself absorb at UV region but has an effect of shifting chromophore peaks to lower wavelength and increases their intensity
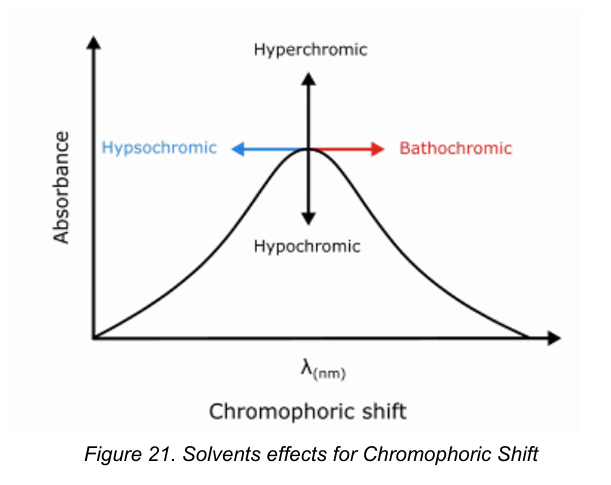
Hypsochromic shift
[Chromophoric Shifts]
The “blue” shift
Transitions are generally shifted to shorter wavelengths with increasing polarity of solvents.
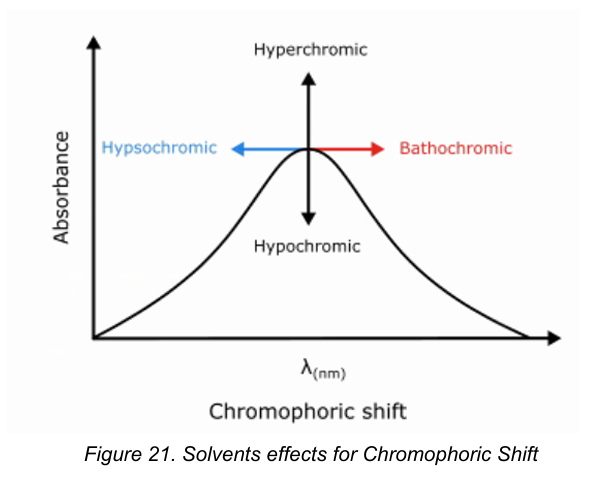
Bathochromic shift
[Chromophoric Shifts]
The “red” shift
Transitions were the peak shifts to longer wavelength with increasing polarity of the solvent.
IR Spectrophotometry
[Type of Spectrophotometry]
electromagnetic radiation ranging between 2500 and 20000 nm is passed through a sample and is absorbed by the bonds of the molecules in the sample causing them to strecth or bend. The wavelength of the radiation absorbed is characteristic of the bond absorbing it.
![<p><span style="color: red"><strong>[Type of Spectrophotometry]</strong></span></p><p>electromagnetic radiation ranging between 2500 and 20000 nm is passed through a sample and is absorbed by the bonds of the molecules in the sample causing them to <strong>strecth </strong>or <strong>bend. </strong>The wavelength of the radiation absorbed is characteristic of the bond absorbing it.</p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/9d915cf0-2d76-4763-941c-81d8bd83783d.png)
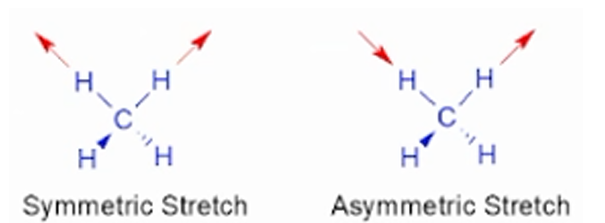
A. Stretching vibration
Involves in the continuous change of interatomic distance along the axis of the bonds between two bonds.
A. Stretching vibration
B. Bending
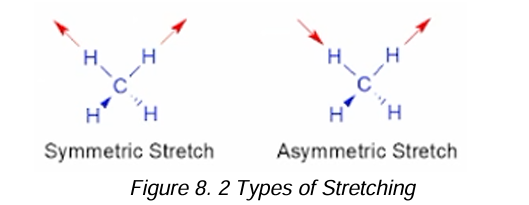
Symmetric
Asymmetric
Types of Stretching Vibration in IR Spectrophotometry [2]
B. Bending
Change on the angle between two bond.
A. Stretching vibration
B. Bending
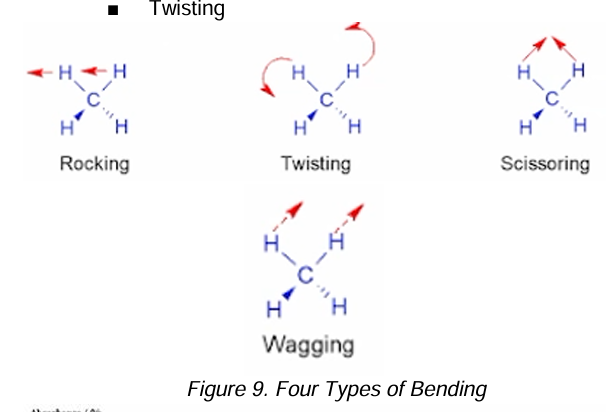
Rocking
Twisting
Scissoring
Wagging
Types of Bending in IR Spectrophotometry [4]
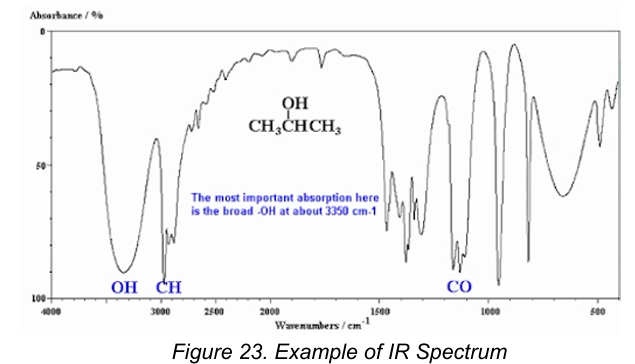
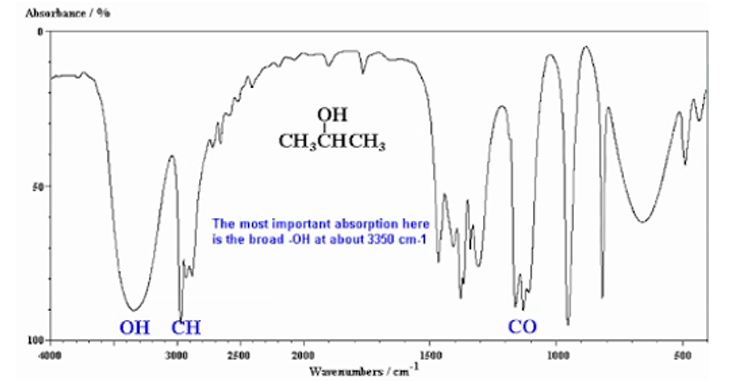
B. IR Spectrophotometry
Interpretation by looking at the peaks and depends of the points stated below:
909-650 cm-1: presence of aromatic structure
1303-909 cm-1: fingerprint region
4000-1300 cm-1: functional group region
The most important is the broad -OH at about 3350 cm-1.
The graph on the picture is an example of what type of spectrum?
A. Fluorescence Spectrophotometry
B. IR Spectrophotometry
C. Molecular Emission Spectrophotometry
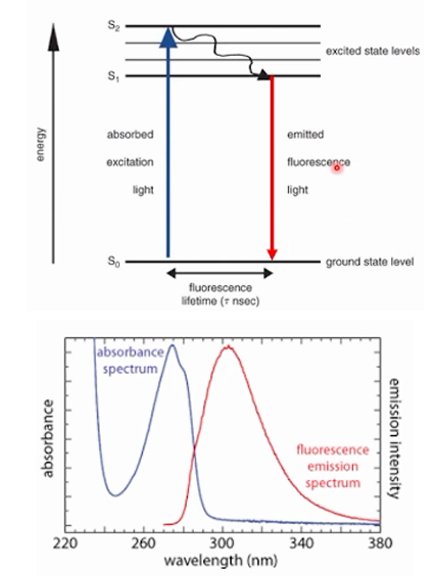
C. Molecular Emission Spectrophotometry
The graph on the picture is an example of what type of spectrum?
A. Fluorescence Spectrophotometry
B. IR Spectrophotometry
C. Molecular Emission Spectrophotometry
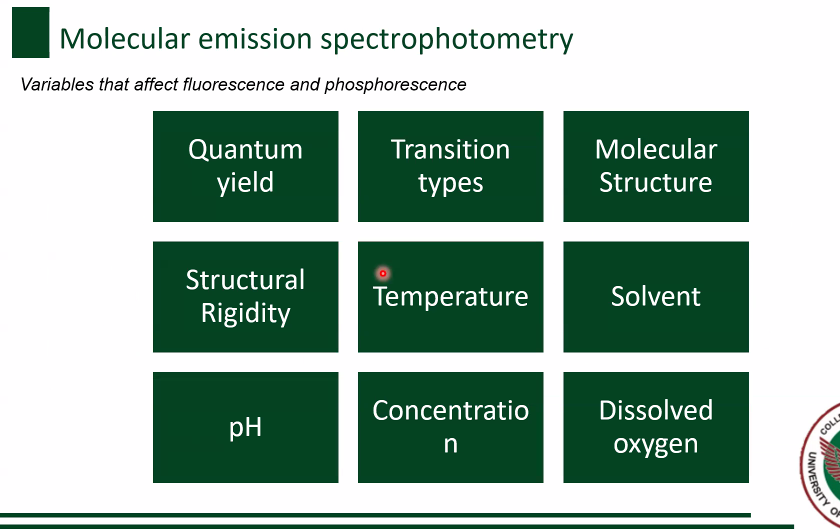
Quantum yield
Transition types
Molecular structure
Structure rigidity
Temperature
Solvent
pH
Concentration
Variables for Fluorescence and Phosphorescence [8]
Quantum yield
[Variable For Fluorescence and Phosphorescence]
also called “Quantum efficiency”
The ratio of the number of molecules that are luminescent to the total number of excited molecules.
For highly fluorescent molecules, the quantum efficiency approaches one. Molecules that do not fluoresce have quantum efficiencies that approach zero.
Fluorescence energy transition
Phosphorescence energy transition
[Variable For Fluorescence and Phosphorescence]
Two Transition types
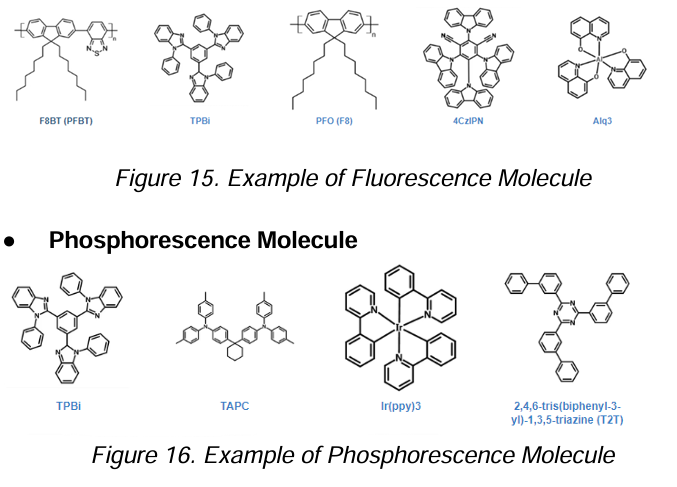
Fluorescence Molecule
Phosphorescence Molecule
[Variable for Fluorescence and Phosphorescence]
Two types of Molecular structure
fluorescence
The explanation for lower quantum efficiency or lack of rigidity is caused by the enhanced internal conversion rate (kic) which increases the probability that there will be radiationless deactivation. Nonrigid molecules can also undergo low-frequency vibration which accounts for small energy loss.
The structural rigidity in a molecule favors [fluorescence/phosphorescence].
Temperature
[Variable of Fluorescence and Phosphorescence]
As the this variable increases, the frequency of the collision increases which increases the probability of deactivation by external conversion.
A. Lower
[Variable of Fluorescence and Phosphorescence]
Solvents with [BLANK] viscosity have higher possibility of deactivation by external conversion.
A. Lower
B. Higher
False.
Fluorescence of a molecule decreases when its solvent contains heavy atoms such as carbon tetrabromide and ethyl iodide, or when heavy atoms are substituted into the fluorescing compound.
[T/F] Fluorescence of a molecule increases when its solvent contains heavy atoms such as carbon tetrabromide and ethyl iodide, or when heavy atoms are substituted into the fluorescing compound.
True
[T/F] The fluorescence of aromatic compounds with basic or acid substituent rings are usually pH dependent.
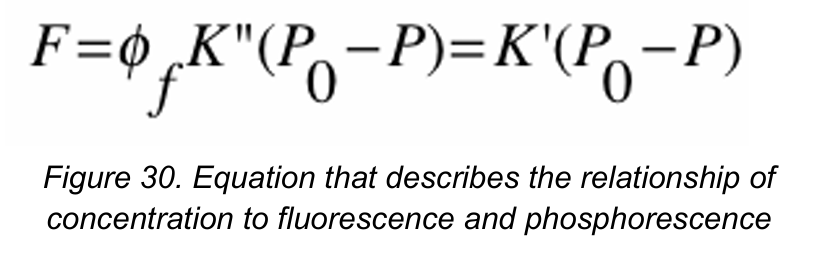
Concentration
[Variable of Fluorescence and Phosphorescence]
The power of fluorescence emission F is proportional to the radiant power is proportional to the radiant power of the excitation beam that is absorbed by the system.
Dissolved oxygen
reduces the intensity of fluorescence in solution, which results from a photochemically induced oxidation of fluorescing species.
B. Non-fluorescent molecule
[Molecular Emission Spectrophotometry]
Excess electronic energy (excited state) converted to vibrational energy (relaxed state) through relaxation
A. Fluorescent molecule
B. Non-fluorescent molecule
A. Fluorescent molecule
[Molecular Emission Spectrophotometry]
Their conversion to vibrational energy is slow, which allows the chance for emission of excess energy to be emitted in the UV / visible region.
A. Fluorescent molecule
B. Non-fluorescent molecule
Those that cannot be detected by atomic absorption spectrophotometry can be detected by molecular emission spectrophotometry.
Molecular emission spectrophotometry is more sensitive than atomic absorption spectrophotometry.
Not for non-fluorescent molecules
Advantage of molecular emission spectrophotometry over atomic absorption spectrophotometry [3]
Excited state
How it emits energy
[Molecular Emission Spectrophotometry]
Two things to measure in fluorescence:
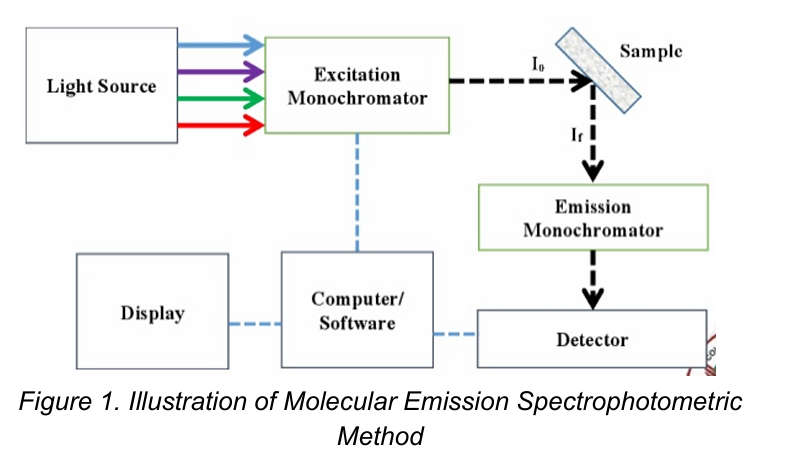
How to determine a substance using molecular emission spectrophotometric method
C. Atomic Absorption Spectrophotometry
Used when you want to determine the presence or limit of heavy metals
A. Atomic Emission Spectrophotometry
B. Mass Spectrophotometry
C. Atomic Absorption Spectrophotometry
D. Light Scattering Spectroscopy / Raman Spectroscopy
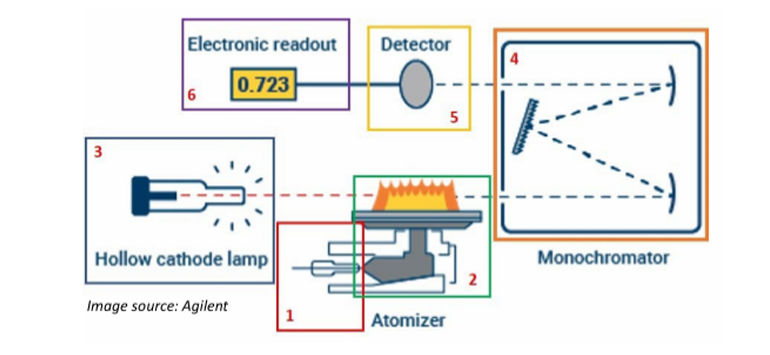
Atomic Absorption Spectrophotometry
Atoms of a metal are volatilized in a flame and their absorption of a narrow band of radiation produced by a hollow cathode lamp, coated with the particular metal being determined, is measured.
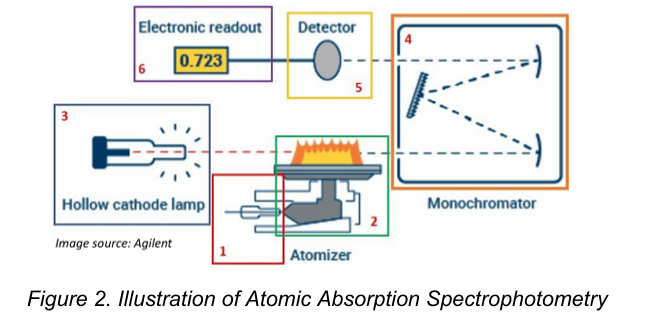
Atomization of the sample
The absorption of radiation from a light source by the free atoms
Process of Atomic Absorption Spectroscopy [2]
D. Light Scattering Spectroscopy / Raman Spectroscopy
Used by geologists to determine ions and elements in rocks and solids.
Used for expeditions and archaeological searches for rocks
A. Atomic Emission Spectrophotometry
B. Mass Spectrophotometry
C. Atomic Absorption Spectrophotometry
D. Light Scattering Spectroscopy / Raman Spectroscopy
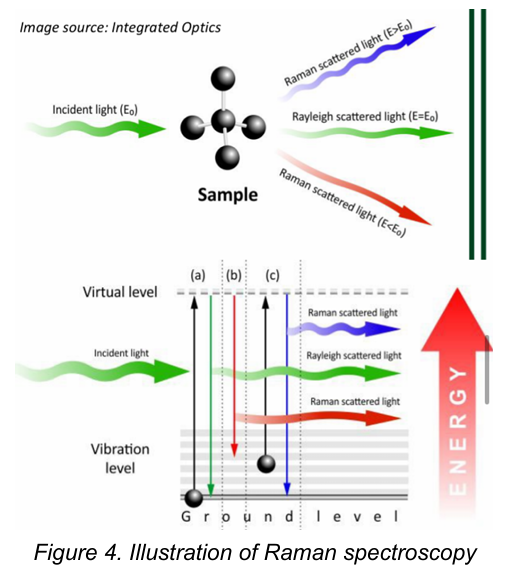
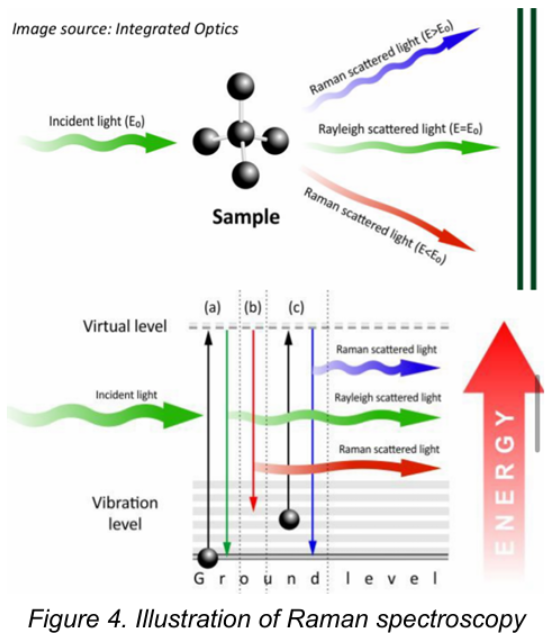
Light Scattering Spectroscopy / Raman Spectroscopy
Most photons are elastically scattered, a process which is called Rayleigh scattering. It is based on the Raman effect, which is the inelastic scattering of photons by molecules.
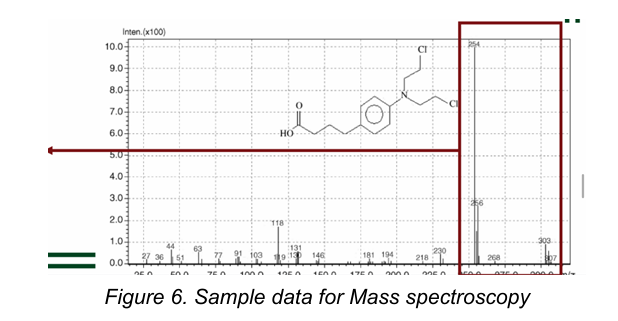
Mass spectrometry
is based on the generation of gaseous ions from analyte molecules, the subsequent separation of these ions according to their mass-to-charge (m/z) ratio, and the detection of these ions.
Electron ionization
The atom or molecule is ionized by knocking one or more electrons off to give a positive ion
Chemical ionization
Ions are accelerated for them to have the same kinetic energy
Electrospray ionization
Deflection of ions by a magnetic field (deflected according to their masses)
Atmospheric Pressure Chemical ionization
The beams passing through the machine is detected electronically, which generates the data (mass of the certain ion)
Stages in applying sample in mass spectrometry: [4]
Determination of drug substances and their metabolites
Therapeutic drug monitoring
Illicit drug analysis
Doping analysis
Drug analysis in pharmaceutical
Applications of Mass Spectrometry [5]
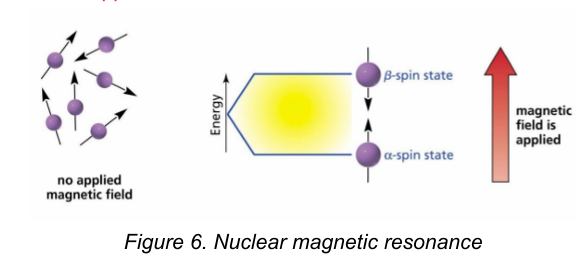
Nuclear Magnetic Resonance (NMR)
is a very valuable technique for the structure elucidation of organic compounds.
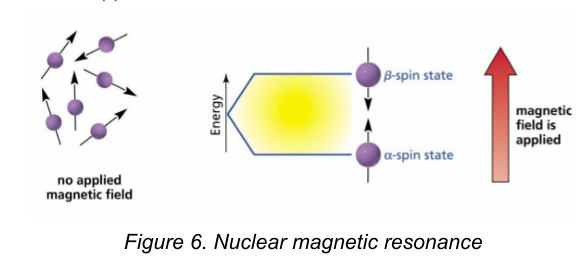
Nuclear Magnetic Resonance (NMR)
Radiation in the radio frequency region is used to excite atoms, usually protons or carbon-13 atoms, so that their spins switch from being aligned with to being aligned against an applied magnetic field. They are not applied to crude drug extracts.
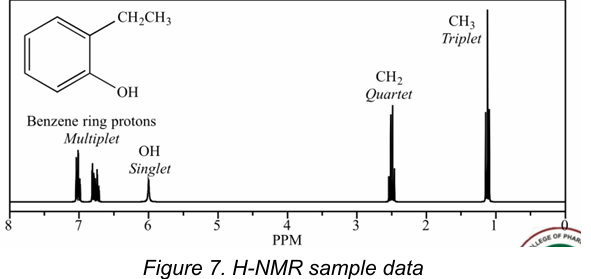
1H-NMR
[Nuclear Magnetic Resonance]
Type of NMR that involves the presence of protons
True
[T/F] Each peak in NMR represents a functional group.
13C-NMR
[Nuclear Magnetic Resonance]
Type of NMR that involves the presence of protons that are Carbon 13 atoms
A.1H-NMR
Type of NMR shown in the picture
A.1H-NMR
B. 13C-NMR
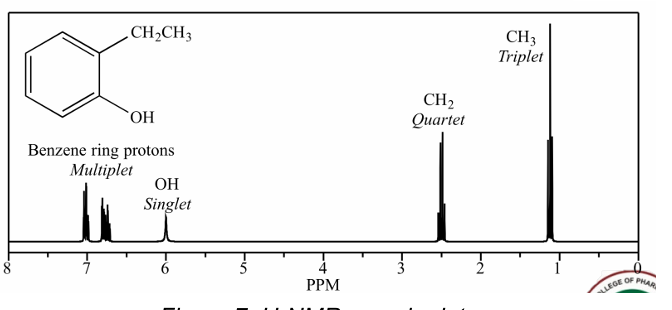
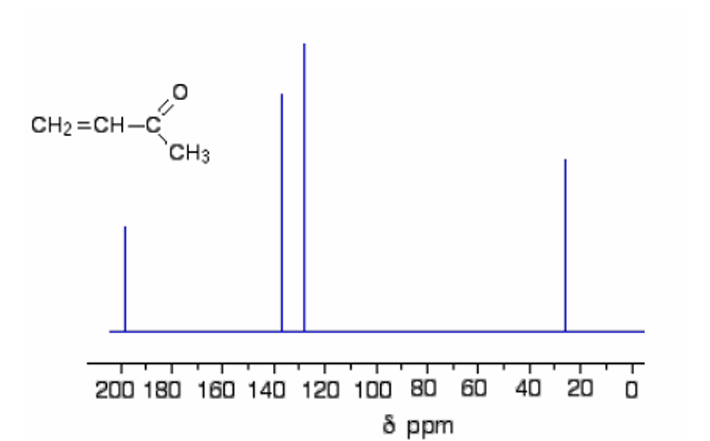
13C-NMR
Type of NMR shown in the picture
A.1H-NMR
B. 13C-NMR
Chromatography
A group of methods used to separate closely related compounds in complex mixtures
The sample is transported in a mobile phase, and the mobile phase is forced through an immiscible stationary phase
Precipitation
Distillation
Extraction
Classical methods of separation in chromatography [3]
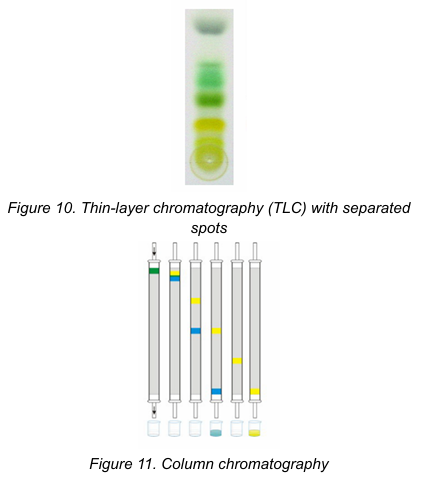
Column Chromatography
Paper Chromatography
Type of Chromatography based on the physical means by which the stationary and mobile phases are brought into contact: [2]
Adsorption chromatography
solute adsorbed on surface of stationary phase
Partition chromatography
Cross-section of open tubular column
Soluble dissolved in liquid phase bonded to the surface of column
Ion-exchange chromatography
Mobile anions held near cations that are covalently attached to stationary phase
Anion-exchange resin; only anions are attracted to it
Molecular exclusion chromatography
Large molecules are excluded
Affinity chromatography
One kind of molecule in a complex mixture becomes attached to a molecule that is covalently bound to stationary phase
All other molecules simply wash through
Type of Chromatography based on the mechanism of interaction of the solute with the stationary phase: [5]