Molecular Geometry and Bonding
1/11
Earn XP
Description and Tags
Flashcards covering various molecular geometries, their bonding groups, shapes, and bond angles.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
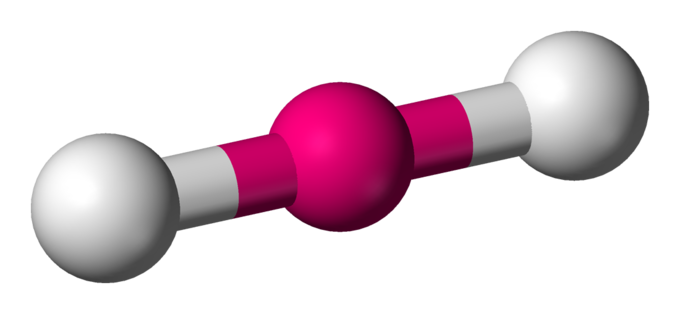
Linear
Molecular shape with 2 bonding groups and a bond angle of 180°.
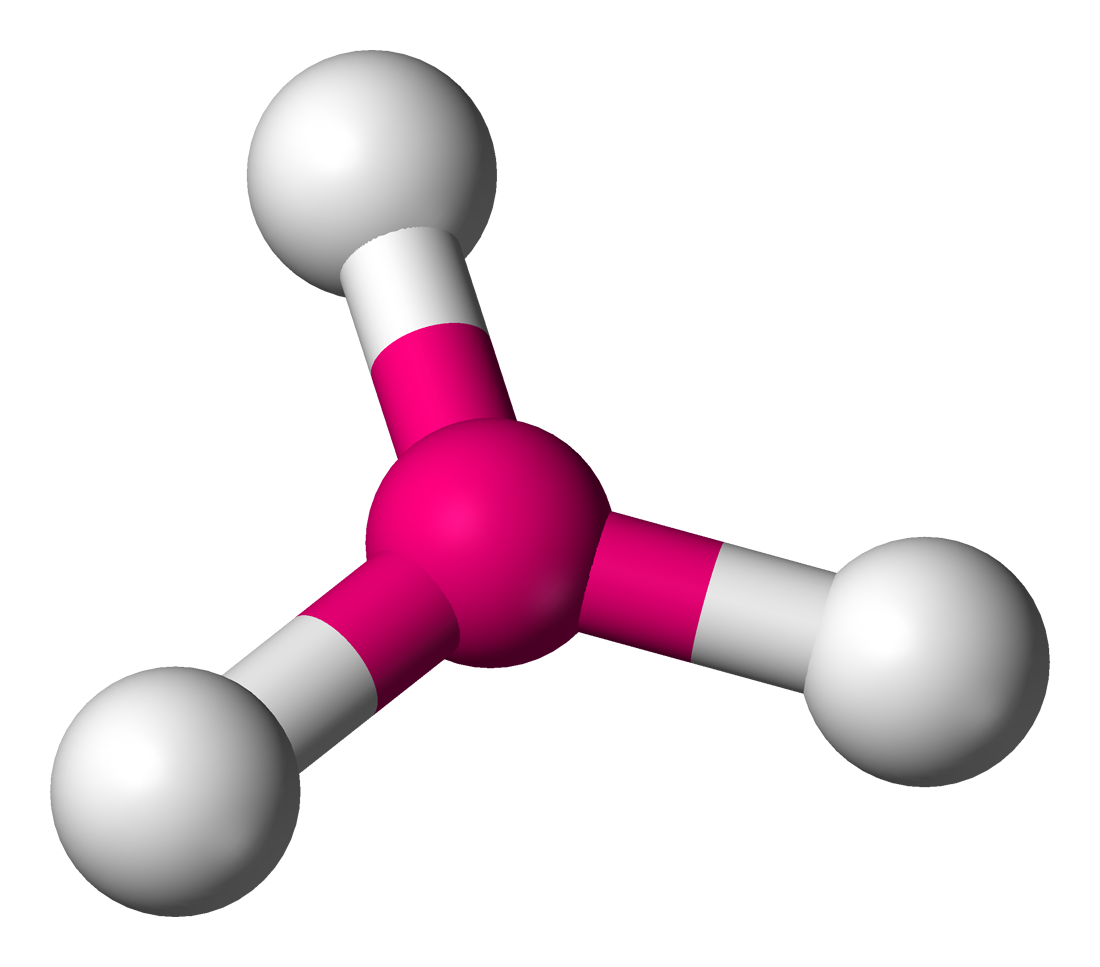
Trigonal planar
Molecular shape with 3 bonding groups and a bond angle of 120°.
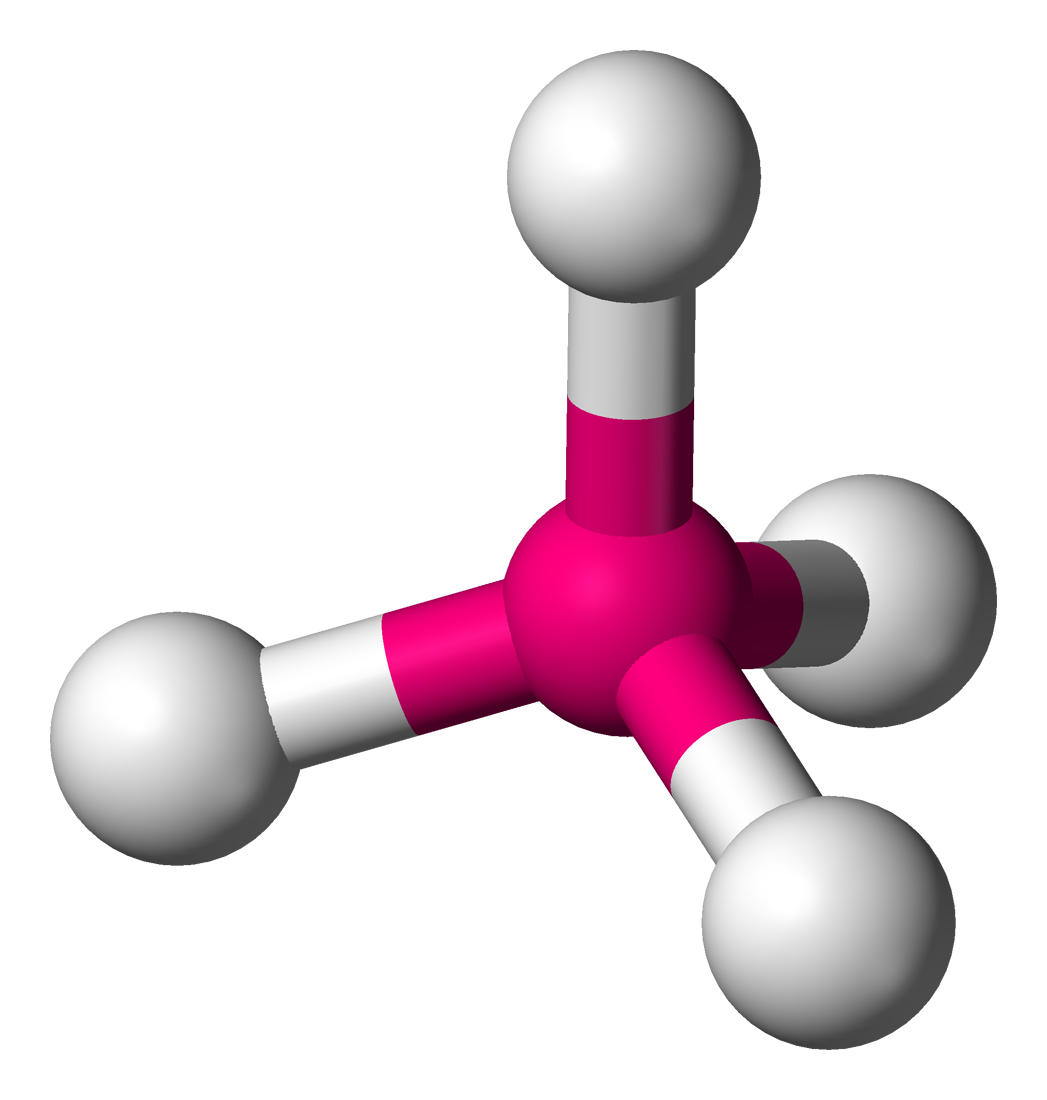
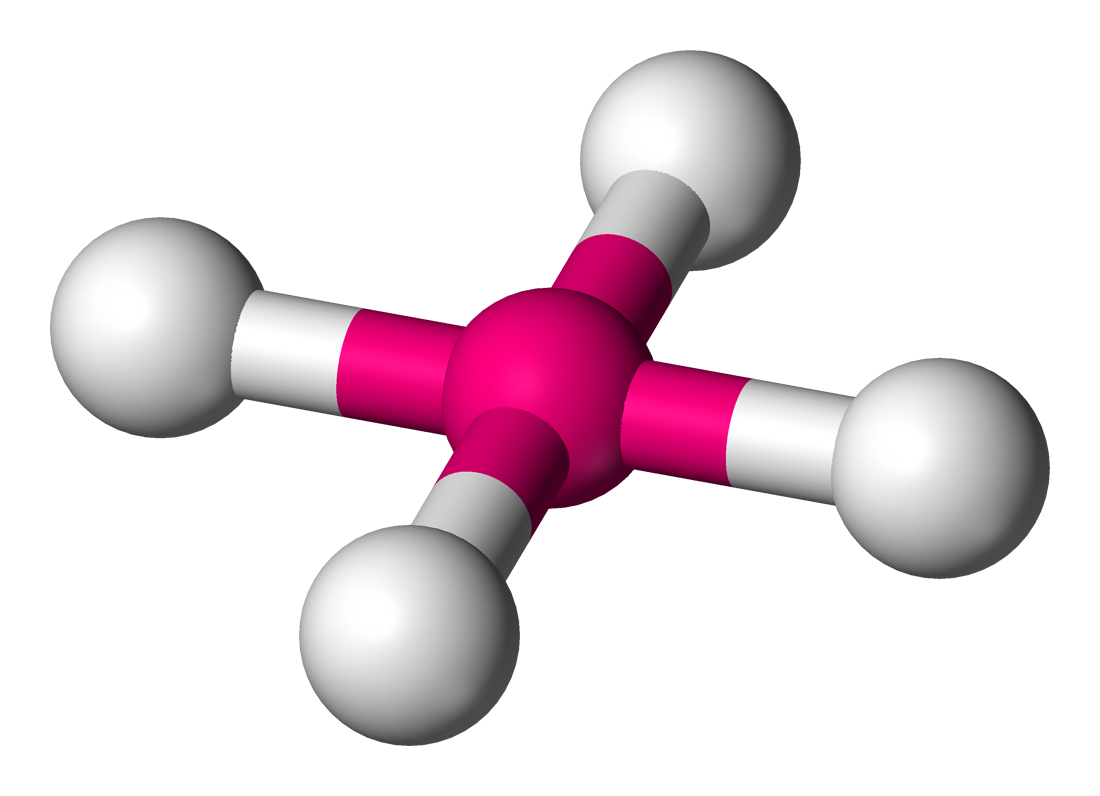
Tetrahedral
Molecular shape with 4 bonding groups and a bond angle of 109.5°.
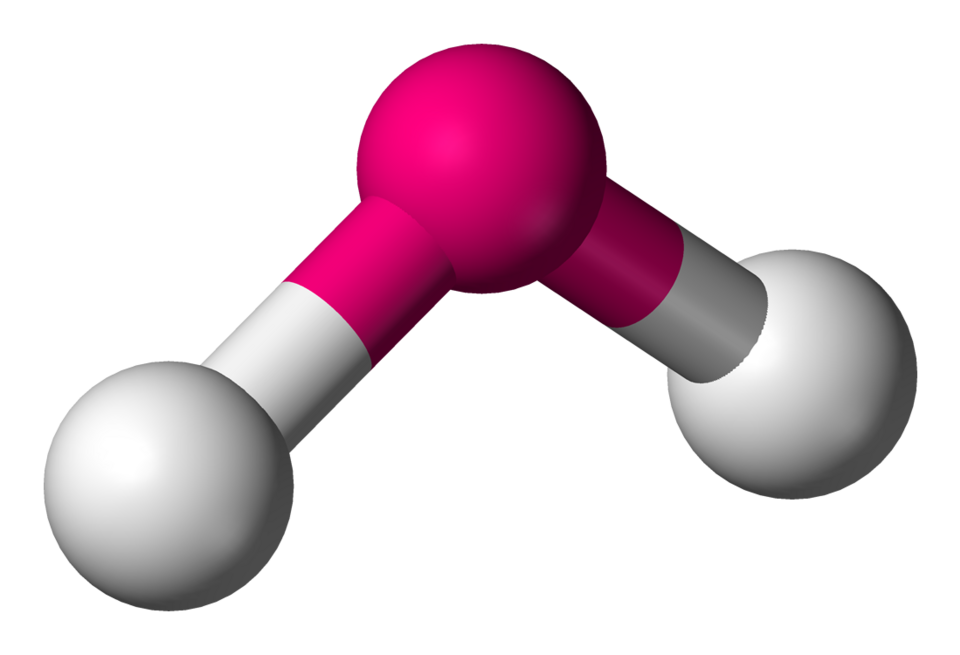
V shaped or bent
Molecular shape with 2 bonding groups and 2 lone pairs, bond angle < 109.5°.
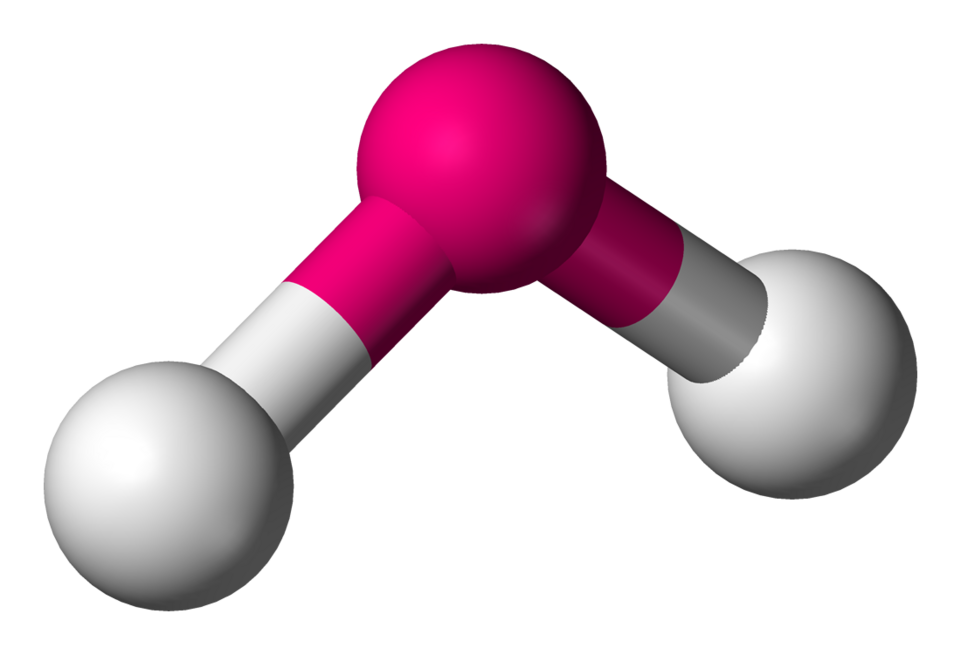
Trigonal bipyramidal
Molecular shape with 5 bonding groups, bond angles of 90° (axial) and 120° (equatorial).
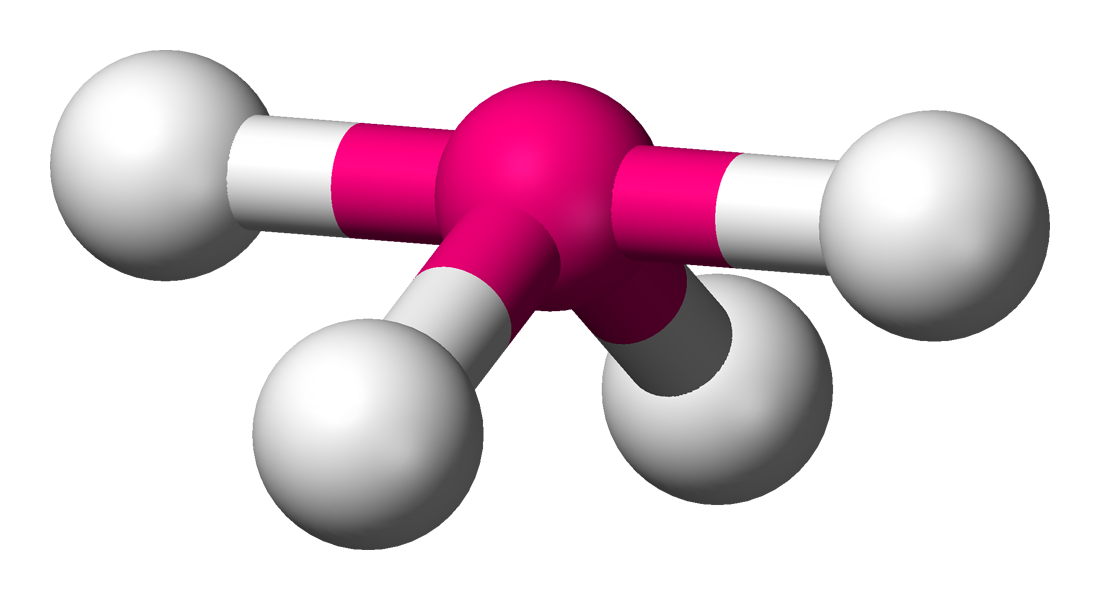
Seesaw
Molecular shape with 4 bonding groups and 1 lone pair, with complex bond angles.

T shaped
Molecular shape with 3 bonding groups and 2 lone pairs, bond angle < 90°.
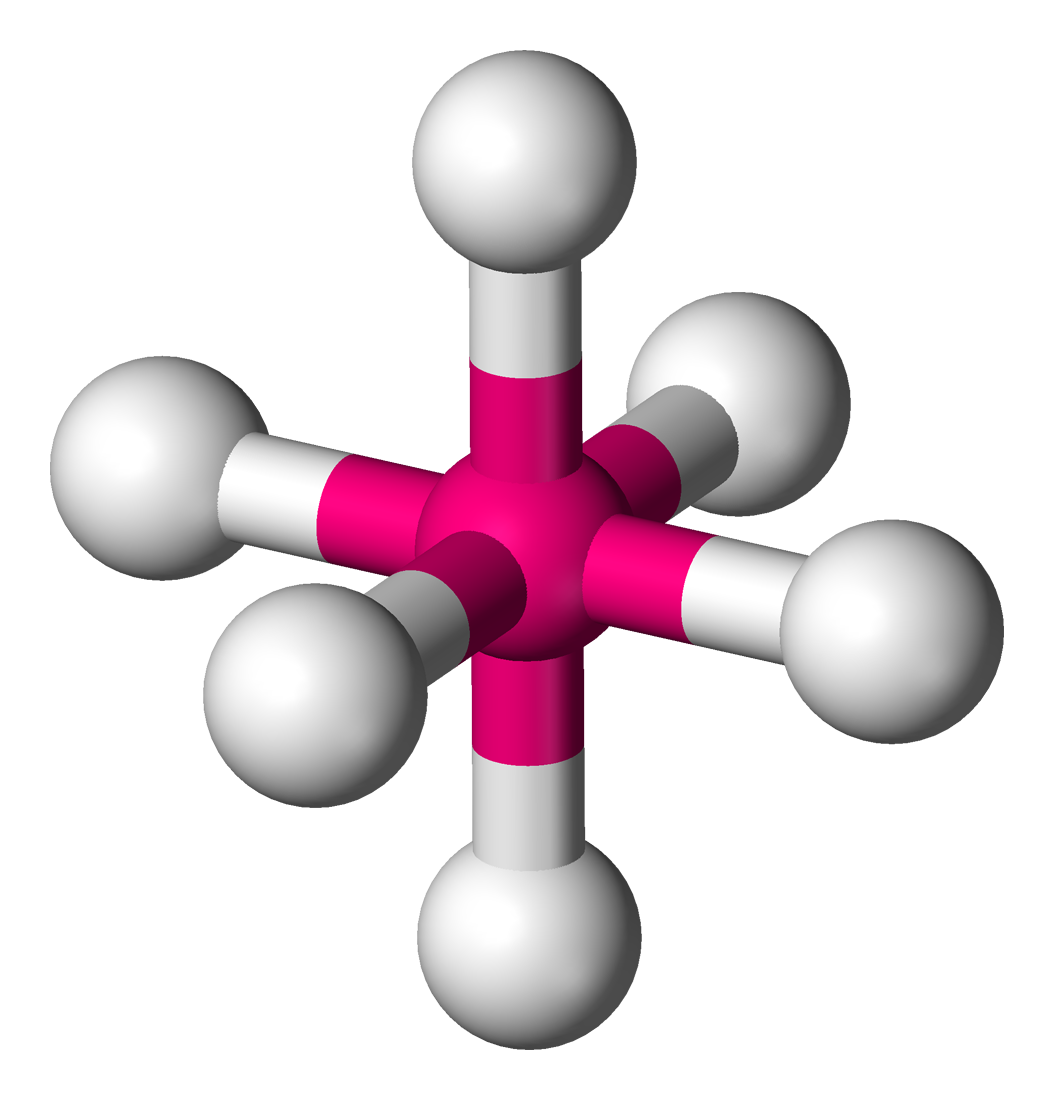
Octahedral
Molecular shape with 6 bonding groups and bond angles of 90°.
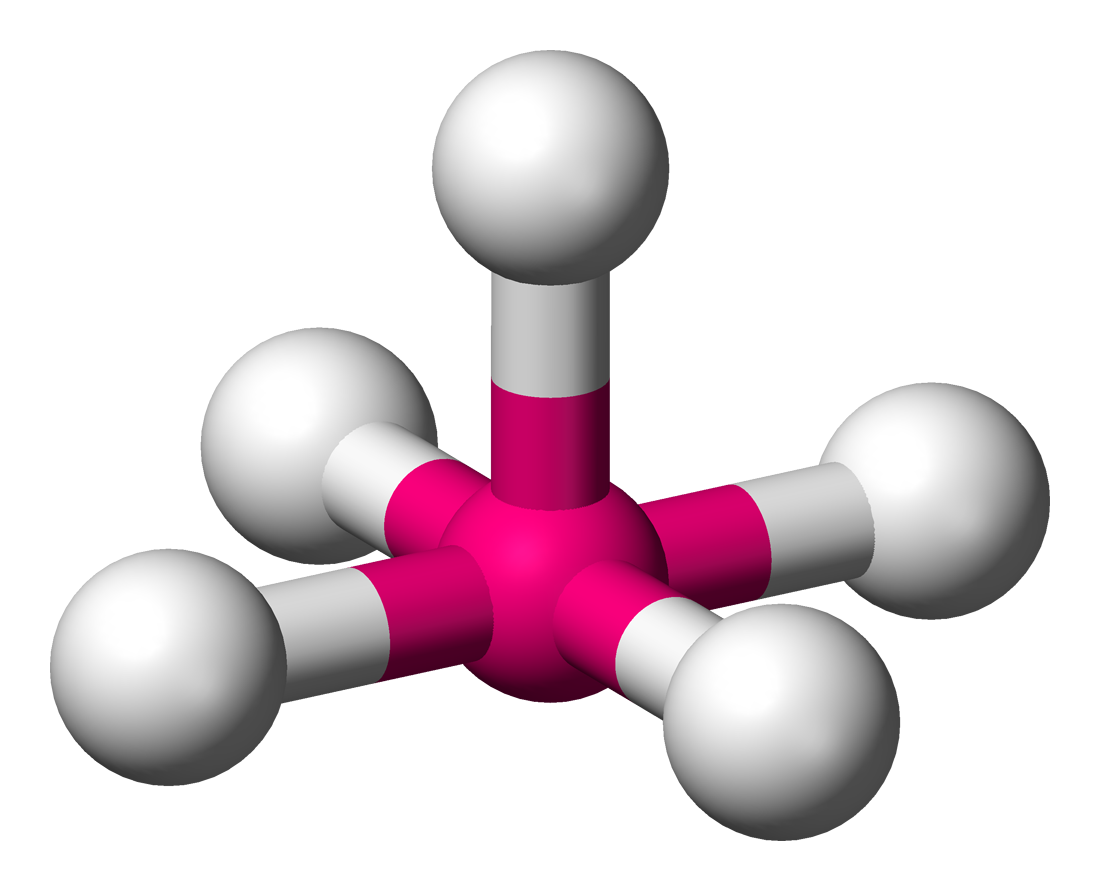
Square pyramidal
Molecular shape with 5 bonding groups and 1 lone pair.
Square planar
Molecular shape with 4 bonding groups and 2 lone pairs.
Square planar with lone electrons
number of bonding groups: 4, number of lone pairs: 2
with bond angle of <120 degrees