molecular orbital theory
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
phase signs of wave functions cause
constructive or destructive interference
interference changes
probability of finding electrons
number of molecular orbitals is always equal to
the number of atomic orbitals that combine (e.g. H2 has two MOs, the bonding and antibonding)
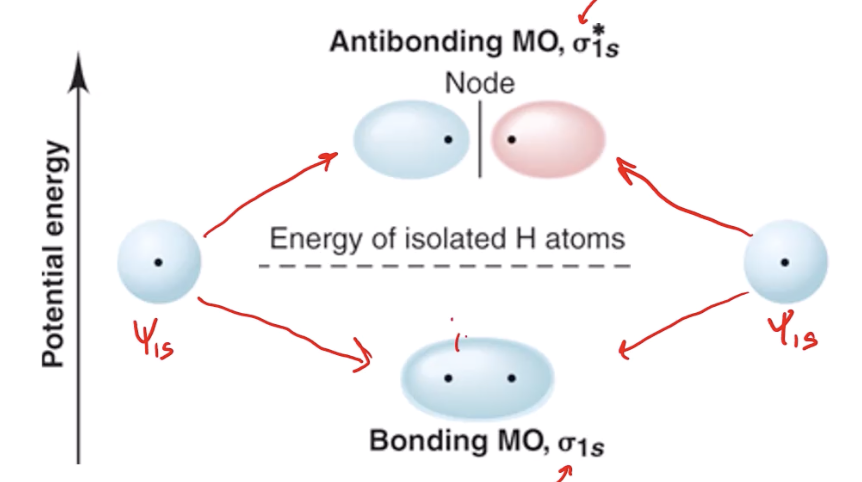
adding in the same phase causes
a bonding orbital, with lower energy than the atomic orbitals
subtracting in opposite phases/adding destructively causes
an antibonding orbital, designated with * - causes a node between the nuclei
bond order =
½ (no of bonding e- — no of antibonding e-)
when doing MO theory of period 2 atoms, can
ignore 1s electrons
bond order can be calculated with
all electrons OR just valence electrons
when doing MO theory move from
bottom to top filling the electrons
resonance
molecules with delocalized electrons that can’t be well described as being in one particular bond; applies to π orbitals