DEFECTS IN CRYSTAL STRUCTURE - BME 296 EXAM 1
1/37
Earn XP
Description and Tags
lectures 3-5
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
38 Terms
defects in crystal structures:
near perfect crystal is difficult to achieve under normal conditions
can either be detrimental or beneficial to physical and mechanical properties of the material
defects often named according to number of dimensions affected by the imperfection
kinds of defects and the dimensions
point defect - 0D
line defect - 1D
planar defect - 2D
volume defect - 3D
why do point defects form
occur due to thermodynamics of crystal growth
during solidification process, vacancy could form due to vibrations
“shake off” atoms from position
creation of defects increases entropy of the system and is therefore favorable
what are the types of point defects: (3)
vacancies, self-interstitials, and impurities
vacancy point defect:
a space where an atom would normally be present but is currently missing in a lattice
Defects are favorable yet produce strain on lattice
Higher temperature more vacancies
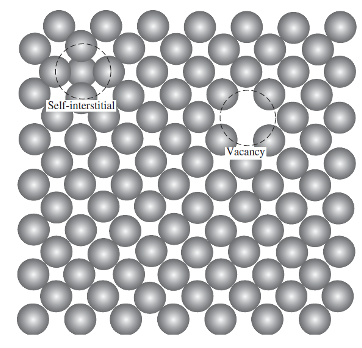
self-interstitial point defect:
occurs when an atom from the crystal is crowded into the interstitial space between two adjacent atoms, occupying a space that would otherwise be empty
equation for number of vacancies:
Nv=number of vacancies
N= number of atomic sites
Q_v = Energy required to form vacancy (Activation energy)
k= Boltzmann's constant (1.38X10-23J/atom-K)
T= absolute temperature
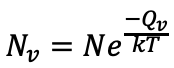
impurity
type of self interstitial defect
type of point defect since they affect the local lattice structure and induce a degree of lattice strain
could be an artifact of material processing or can be added deliberately to alter the final properties of the material
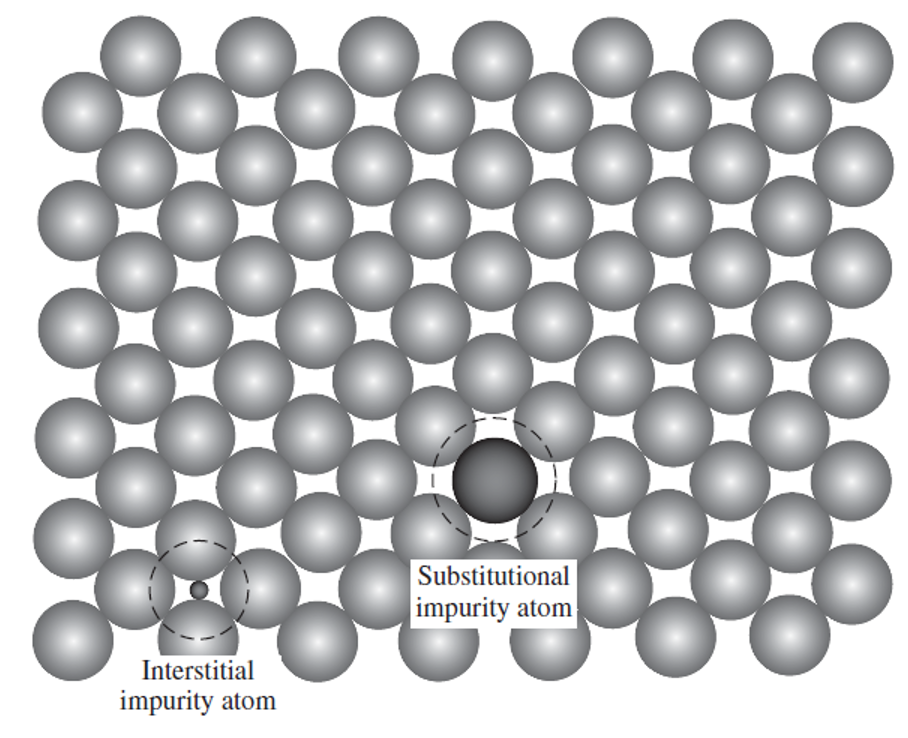
two types of impurities:
Host material =solvent, impurity =solute.
The solute atoms can either fill spaces between the solvent atoms (interstitial solution) or take the place of the solvent atoms (substitutional solution)
more on impurities:
Not always possible to predict when interstitial or substitutional solutions will be formed
Interstitial solutions occur when the solute atom is smaller than the solvent, placement in the interstitial spaces without extreme lattice strain.
In alloys, the impurity atom has been added in a particular concentration to improve properties of the host material.
Alloys used to increase strength, impart corrosion resistance ,or improve electrical properties of a pure metal.
Common interstitial solid solution alloys include steels (alloys of carbon and iron), which form the basis of stainless-steel orthopedic implants.
how are metals manufactured?
molten metal is first cooled, followed by shaping or pouring into a mold for shaping
each of these steps matter as they determine mechanical properties of the material
formation of crystals occurs in all axes simultaneously when allowed to freely cool
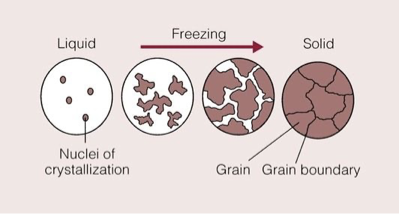
process of crystal formation and grain boundary:
First nucleation occurs where single unit cell is formed
New unit cells attach to existing unit cell in growth phase
As crystals meet grain boundaries are formed
As crystals are forming unit cells will usually align in patterns
what is a line defect also known as
edge dislocation
why do line defects occur?
when an extra portion of a plane of atoms (half-plane) terminates in a crystal
the defect is the line that defines the end of the extra half-plane, called the dislocation line
occurs due to accidents in the crystal growth process, internal stresses from other defects in the crystal
Two important characteristics of dislocations are the magnitude and direction of atomic displacement
difference between point defect and line defect/edge dislocation?
point - at or around a single point of the crystal lattice
line - plane of an atom
how do line dislocations affect properties?
plastic deformation occurs by slip where an edge dislocation slides over adjacent plane of half-line atoms
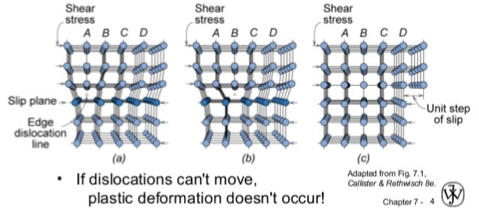
line defects increase what?
PLASTICITY
energy around atoms
Atoms at the surface of a material are not bonded to the maximum possible number of nearest neighbors, so they possess higher energy than those atoms located inside a crystal
This extra energy is called the surface free energy or surface tension on and is expressed in units of energy per unit area
The existence of these sites with higher energy is thermodynamically unstable, and the need to minimize surface energy leads to chemical reactions at the surface
what do planar defects create?
grain boundaries
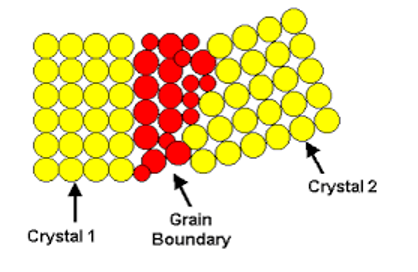
grain boundaries in planar defects
Most metals and ceramics are polycrystalline
The interface between these grains is called the grain boundary.
Grain boundary atoms are in a higher energy state than comparable atoms in the center of the grain.
This leads to higher chemical reactivity in these regions.
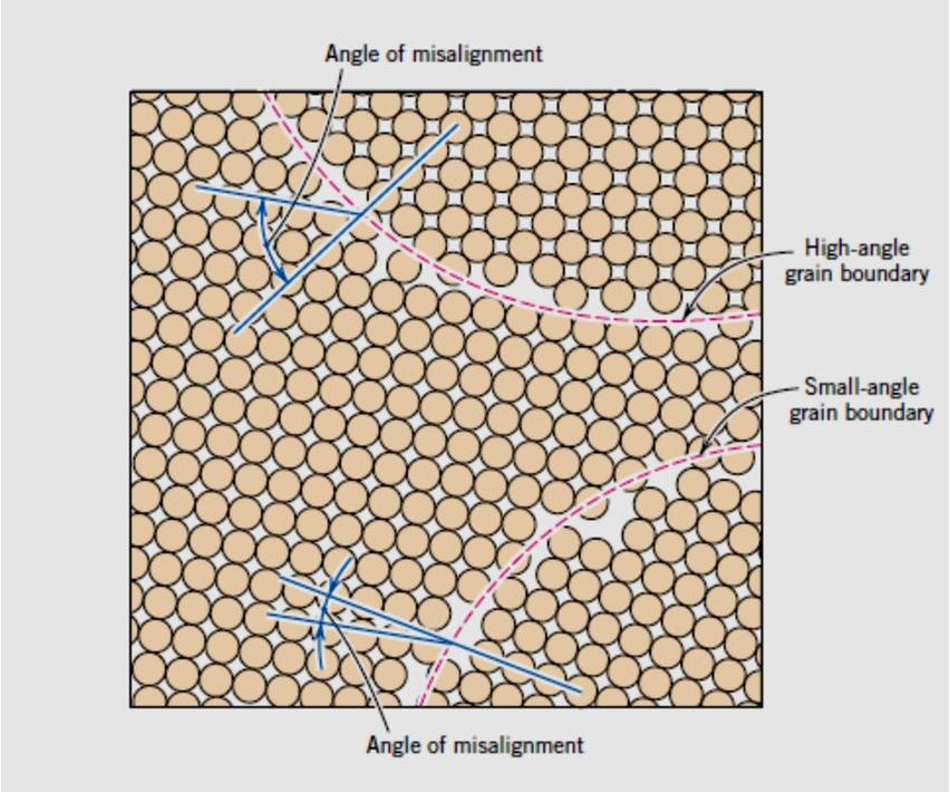
grain boundaries
There are two types of grain boundaries that can be distinguished via microscopic observation.
If the grains on either side of the boundary have similar orientations (only a few degrees different), this is a small-angle grain boundary
Grains with more severe misalignment form high-angle grain boundaries.
The greater the degree of misorientation, the higher the energy of the boundary.
volume defects
Volume defects are three-dimensional regions in which the long-range order of the crystal is lost.
Examples of volume defects include precipitates and voids.
Precipitates are clusters of substitutional or interstitial impurities, while voids are three-dimensional aggregates of vacancies
Voids important in biomaterials, it is possible to create void space (pores) in a controlled manner to alter the biological response to the material.
diffusion
Solid state diffusion as it takes place in solid materials
High concentration to low concentration
Impurity diffusion
Self diffusion
Requirements for diffusion:
Vacant space
Energy to break bonds with neighboring atoms (from atomic vibrations when temperature of material is more than 1K; low temp.. low vibration.. may not break bonds)
Increase temp increase rate of diffusion
diffusion mechanisms
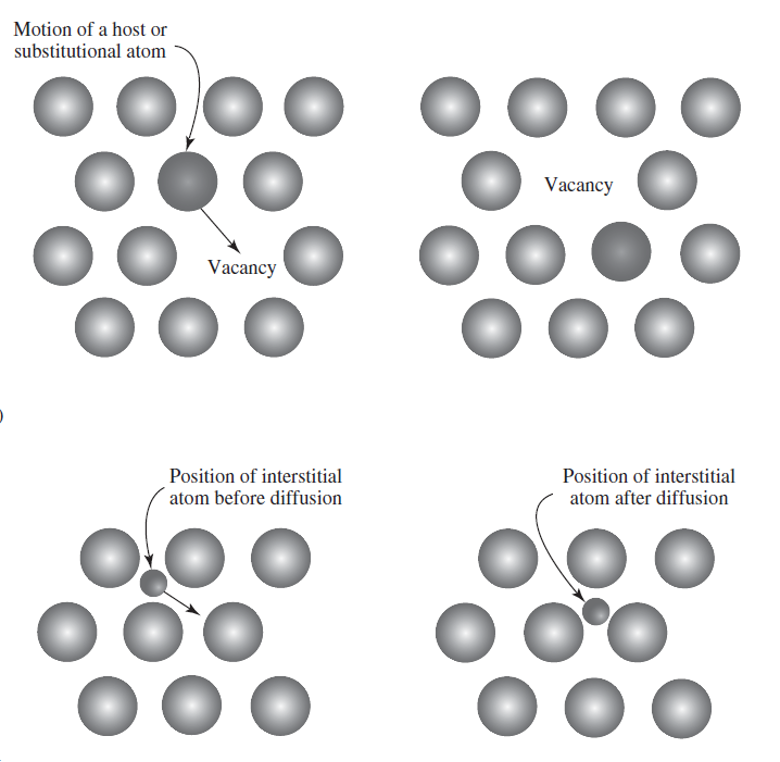
Two main types of diffusion occur in metals- vacancy and interstitial diffusion.
Vacancy: Atom jumps to an adjacent vacancy, thereby exchanging the location of the atom and the vacancy
the direction of atomic diffusion is opposite to that of vacancy diffusion.
Interstitial diffusion: an atom migrates from one interstitial position to a neighboring position
This usually occurs only with small atoms such as hydrogen, carbon, nitrogen and oxygen that can easily fit into the interstitial spaces.
Because of the small size of the diffusing species and their increased mobility, interstitial diffusion generally occurs more rapidly than vacancy diffusion.
how can we make metals stronger despite dislocations?
strain hardening and annealing
strain hardening
Ductile materials are deformed below their melting point causing an increase in number of dislocations (increased density)
distance between dislocations is reduced and this blocks motion
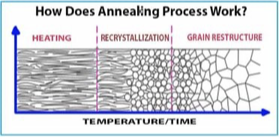
annealing
Increase temperature causing increased diffusion which causes dislocations to start moving
relieves strain energy thereby reducing dislocations
ceramics
two or more elements
can have ionic or covalent bonds (mainly ionic i think)
more ions than atoms
properties of ions
cations must be next to anions; maximize number of nearest neighbors that are anions
anions and cations must contact each other for a stable structure
what are cations and anions?
cation has positive charge, anion has negative charge
what are the sizes of cations and anions and why are they different?
cations are smaller because loss of electron will cause the positively charged nucleus to have greater attraction to remaining electrons.
anions have large electron cloud which will repel each other and less attractive force between positively charged nucleus and electron cloud.
crystal structure in ceramics
affected by two parameters:
The magnitude of the electrical charge on the constituent ions (important to keep crystal electrically neutral)
The physical size of these ions (need to know radii of both anion and cation) (Rc/Ra must be <1)
Crystal structures in ceramics exist in such a way that they satisfy charges (neutral) and size where (Rc/Ra<1)
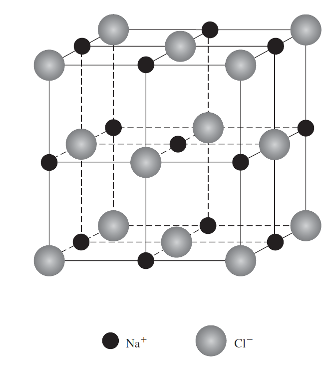
AX crystal structures
Both the cation and anion have the same charge, an equal number of each is required for a stable crystal structure.
A representing the cation and X representing the anion.
Example: Sodium chloride
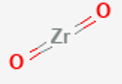
AmXp crystal structures
Cations and anions that do not have equal charges, leading to compounds with the formula AmXp where m and/or p≠1
Example: Zirconia, ZrO2
Ceramics can also have more than one type of cation leading to AmBnXp
Such combinations are mostly found in materials for biomedical applications since we add several different elements to improve properties.
point defects is crystal structures - ceramics
Both interstitial and vacancy point defects can occur for either the cation or anion.
Defect should not affect the electroneutrality of the material.
individual point defects do not occur since that would leave the crystal with a net charge
Group defects occur so neutrality can be maintained.
Example of a group defect is Schottky defect and Frenkel defect.
Schottky defect
vacancies in both cations and anions in the correct ratio to maintain neutrality
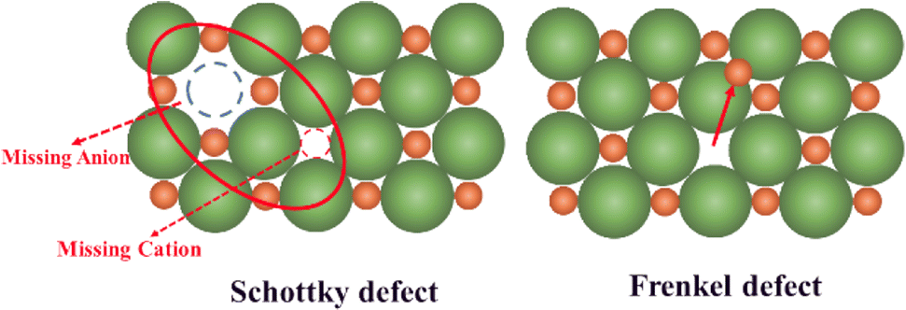
Frenkel defect
a vacancy/interstitial pair is created to maintain electroneutrality
only occurs with cations, since anions are too large to reside in the interstitial space of the crystal without a significant amount of lattice strain.
REVIEW SLIDE
Metals
Metallic bonds
APF, miller indices
Line deformation- plasticity
Small angle, large angle grain boundary
Usually orthopedic implants
Ceramics
Ionic bonds, electroneutrality most important
Brittle- no plasticity due to ionic bonds
Dentistry, coating for orthopedic implants