Cellular Respiration
1/77
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
78 Terms
What is the overall oxidative, exergonic process (△G = -686 kcal/mol) that breaks down glucose in order to derive energy in the form of ATP?
cellular respiration
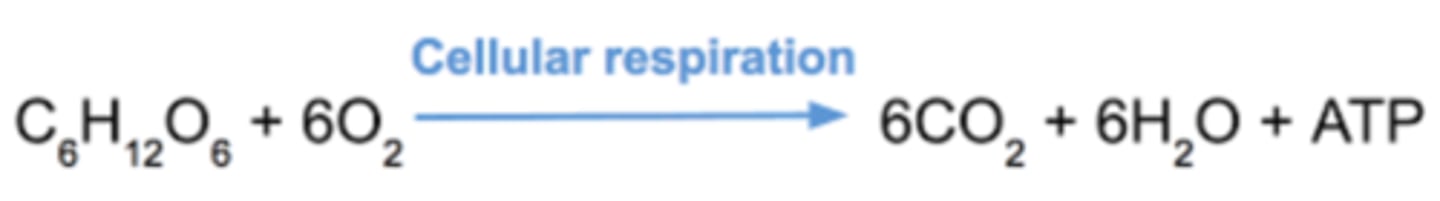
What is the overall reaction of cellular respiration?
C6H12O6 + 6O2 → 6CO2 + 6H20 + ATP
What are the 4 major steps of cellular respiration?
1. glycolysis
2. pyruvate decarboxylation
3. citric acid cycle
4. electron transport chain
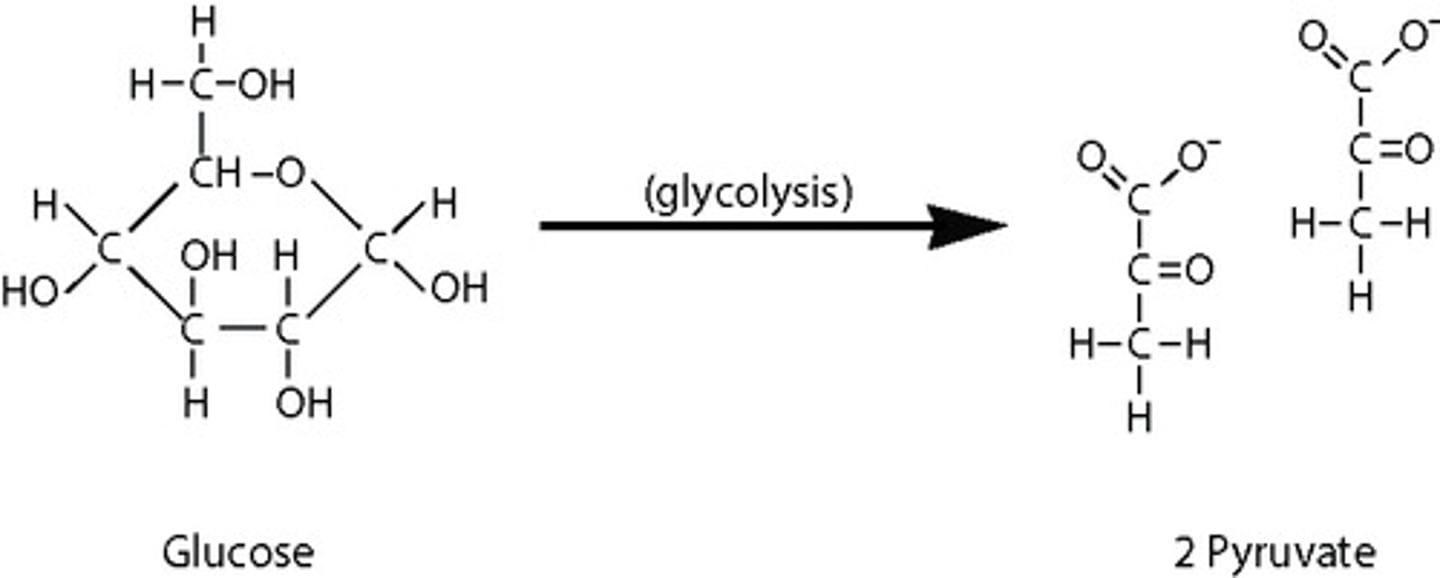
What is the decomposition of glucose into pyruvate within the cytosol?
glycolysis
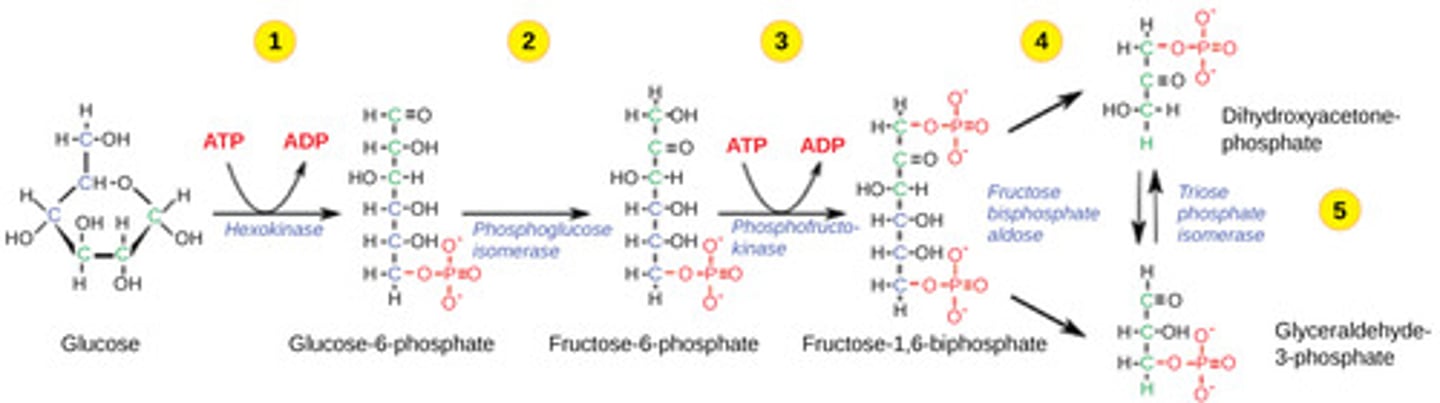
How much energy investment is required for glycolysis?
2 ATP
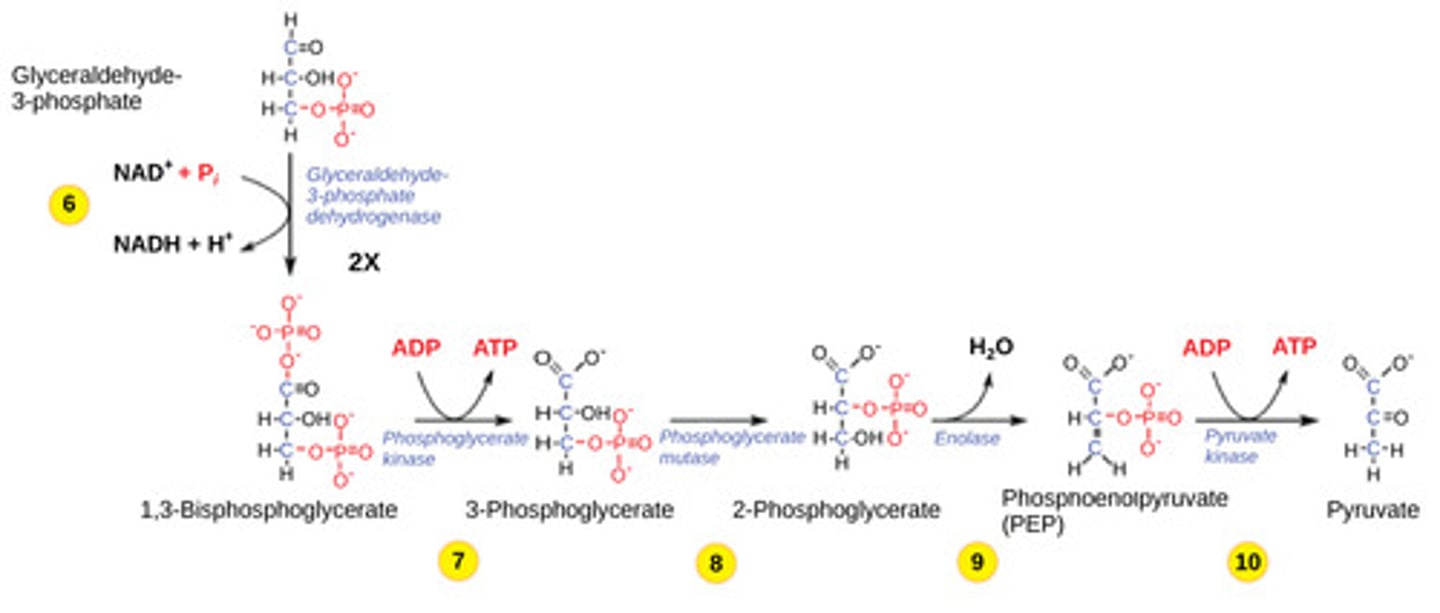
What are the products from glycolysis?
2 NADH + 4 ATP + 2 pyruvate
(Note: 4 ATP produced - 2 ATP invested = 2 ATP net)
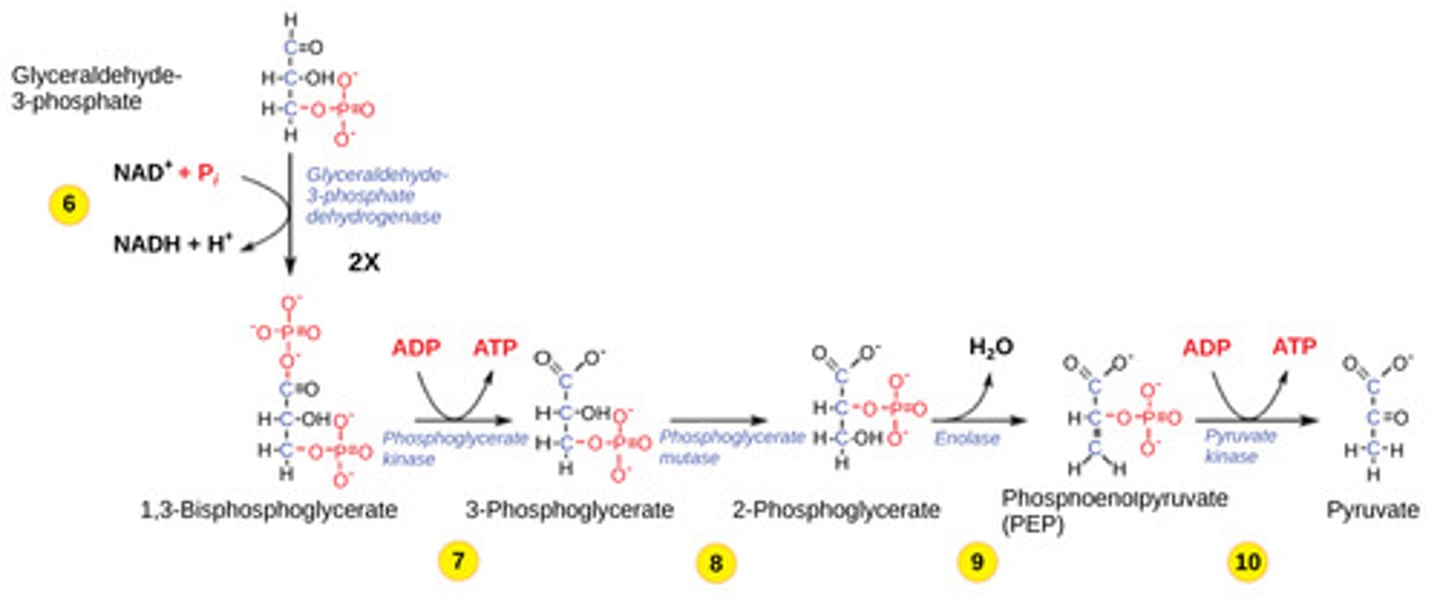
The ATP formed during glycolysis is created via what process?
substrate level
phosphorylation
(Note: direct transfer
of phosphate to ADP)
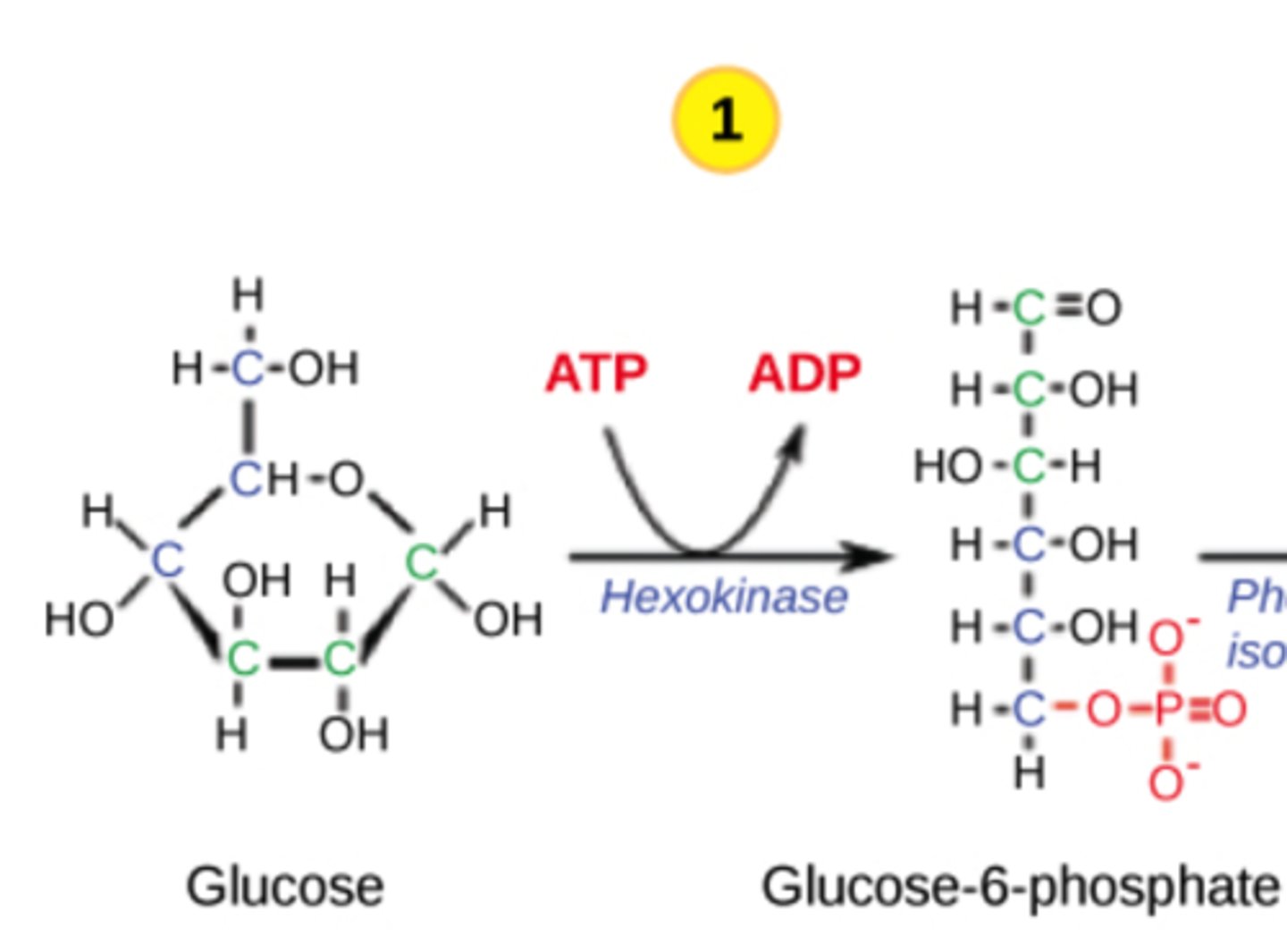
Which step in glycolysis is the first irreversible step?
hexokinase
(Note: step 1)
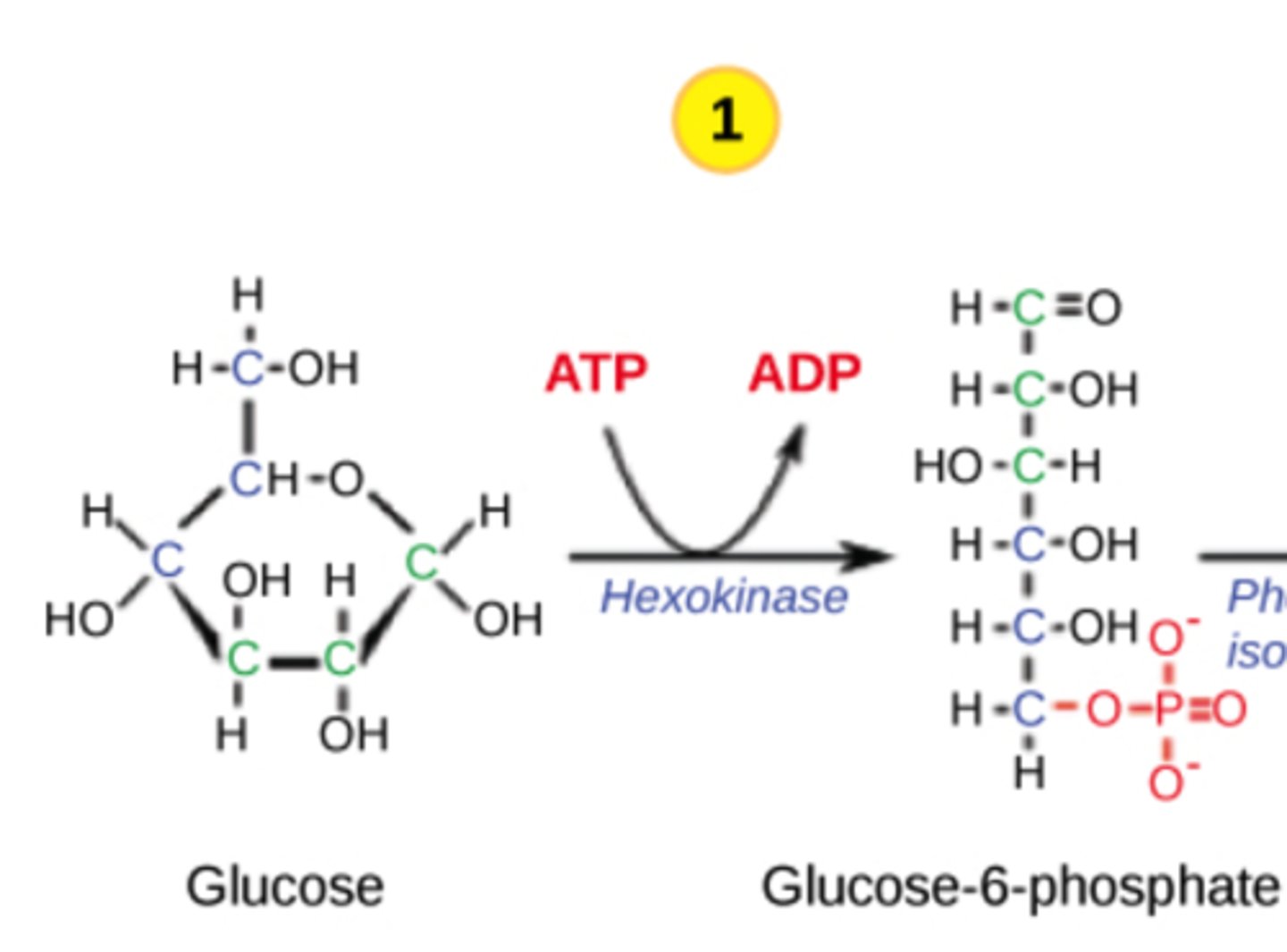
Why is the hexokinase step irreversible?
it phosphorylates
glucose, which
traps it in the cell
Which step in glycolysis is the second irreversible step?
phosphofructokinase (PFK)
(Note: step 3)
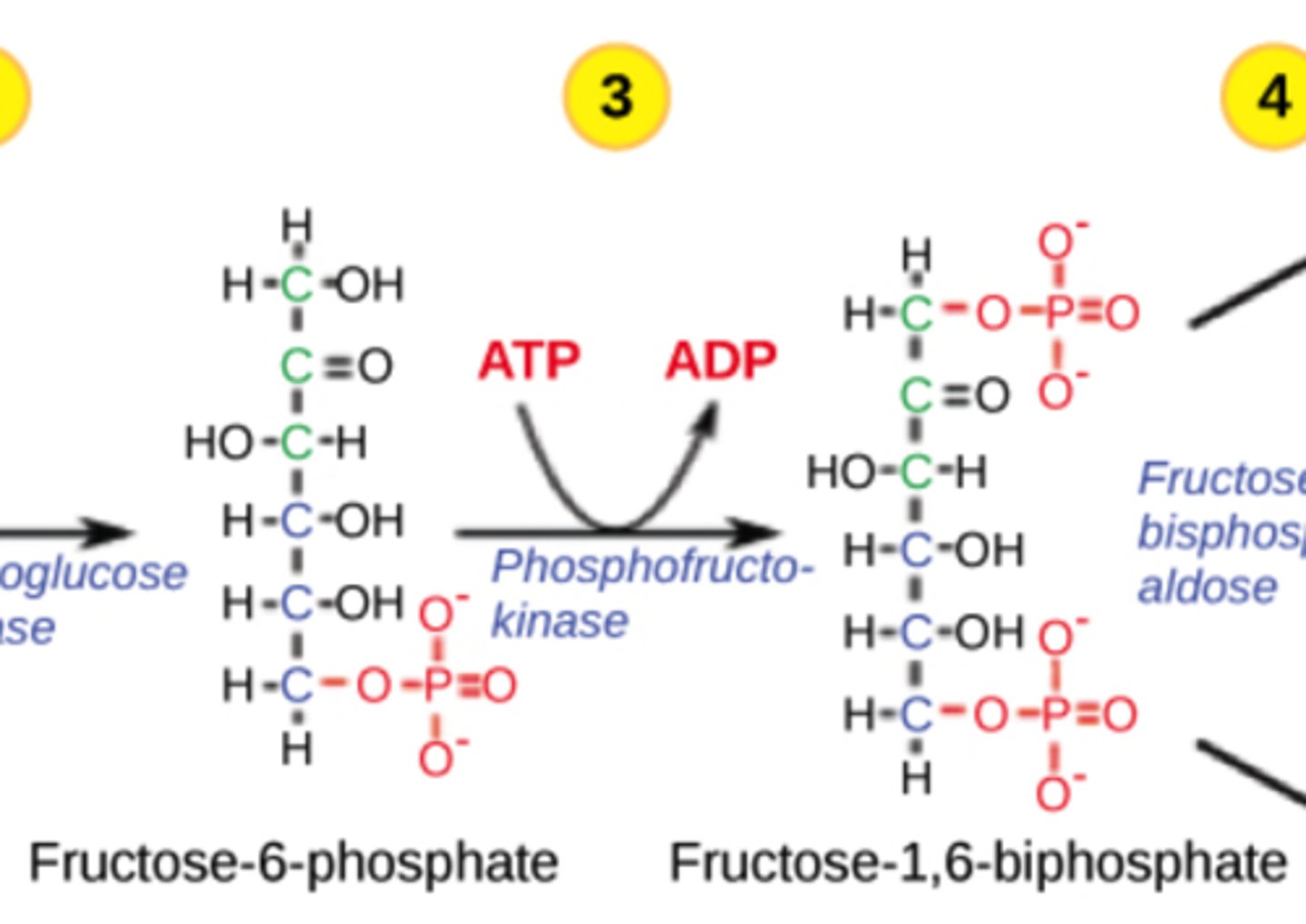
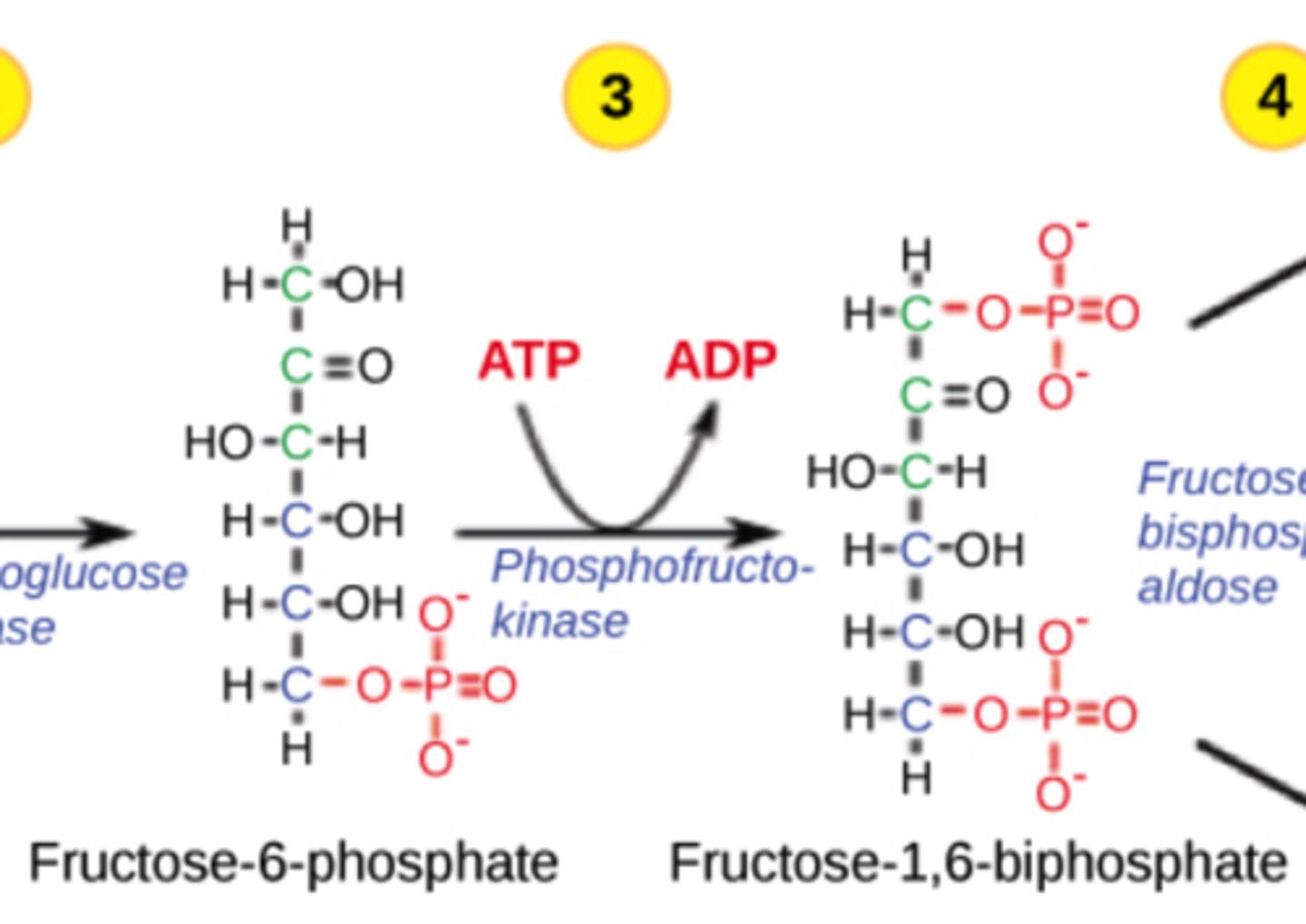
Why is the PFK step irreversible?
adds a second phosphate, forming
fructose 1,6-bisphosphate,
which is energetically favorable
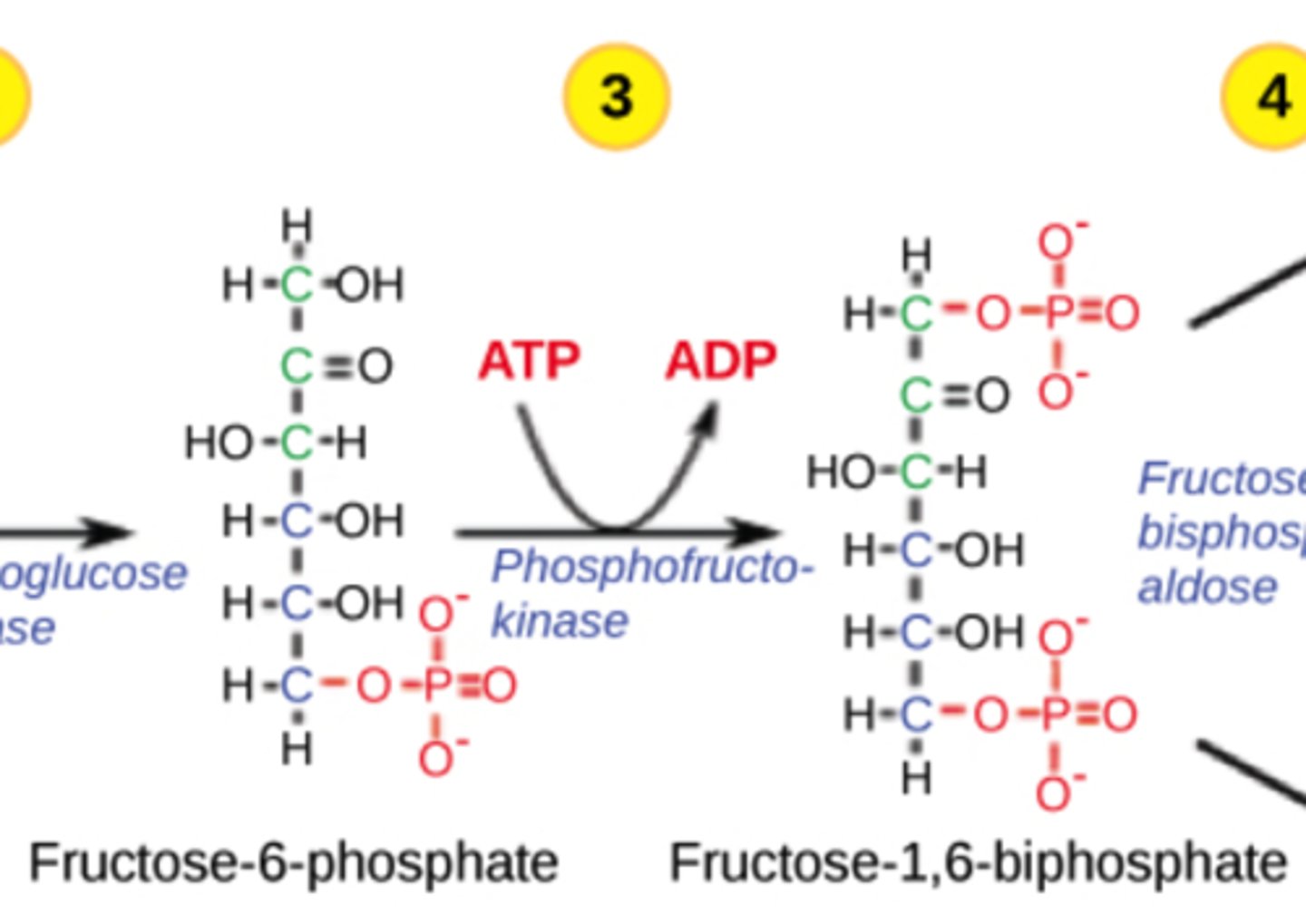
Which step in glycolysis is the major regulatory point?
PFK
(Note: allosteric regulation)
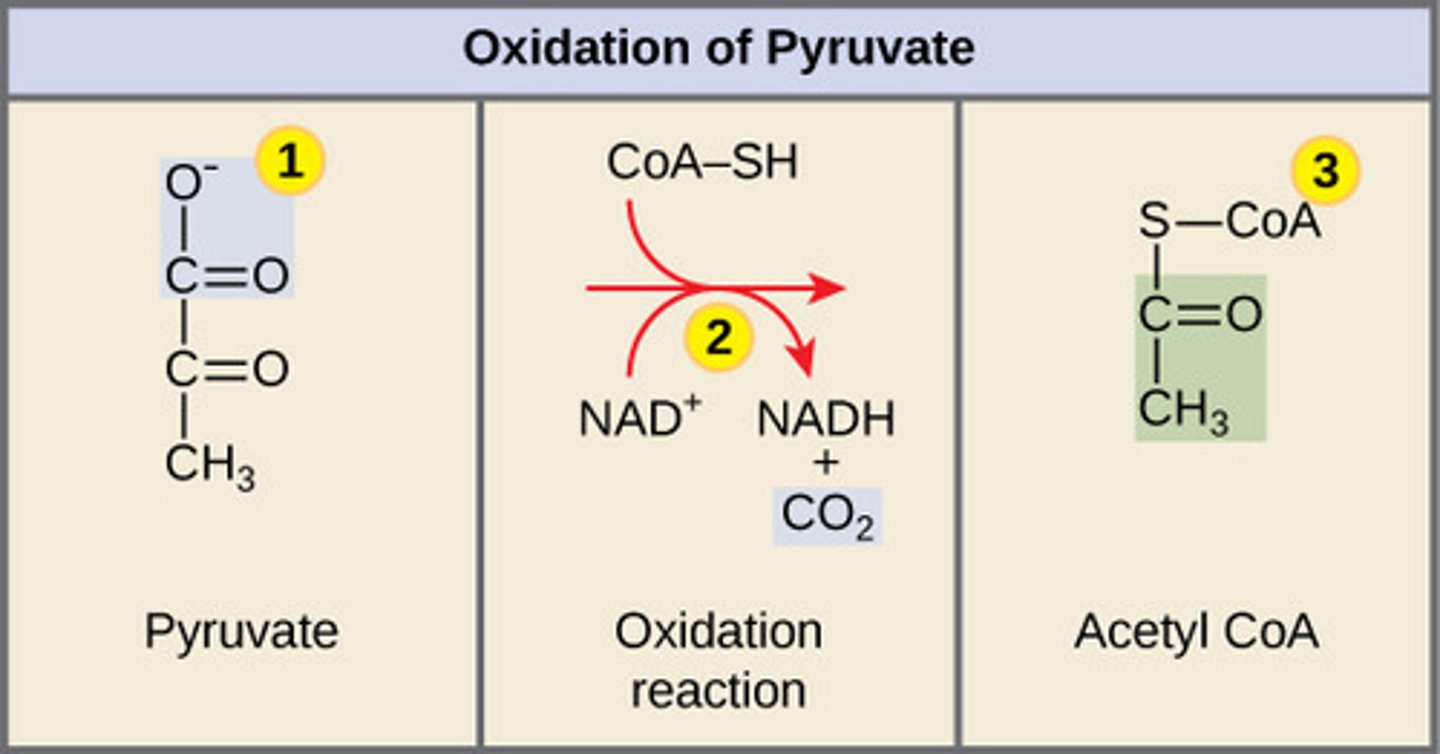
Where does pyruvate decarboxylation take place?
mitochondrial matrix
In pyruvate decarboxylation, what are the products for every molecule of glucose entered?
2NADH + 2 CO2 + 2 Acetyl CoA
(Note: 2 pyruvate
molecules per
glucose molecule)
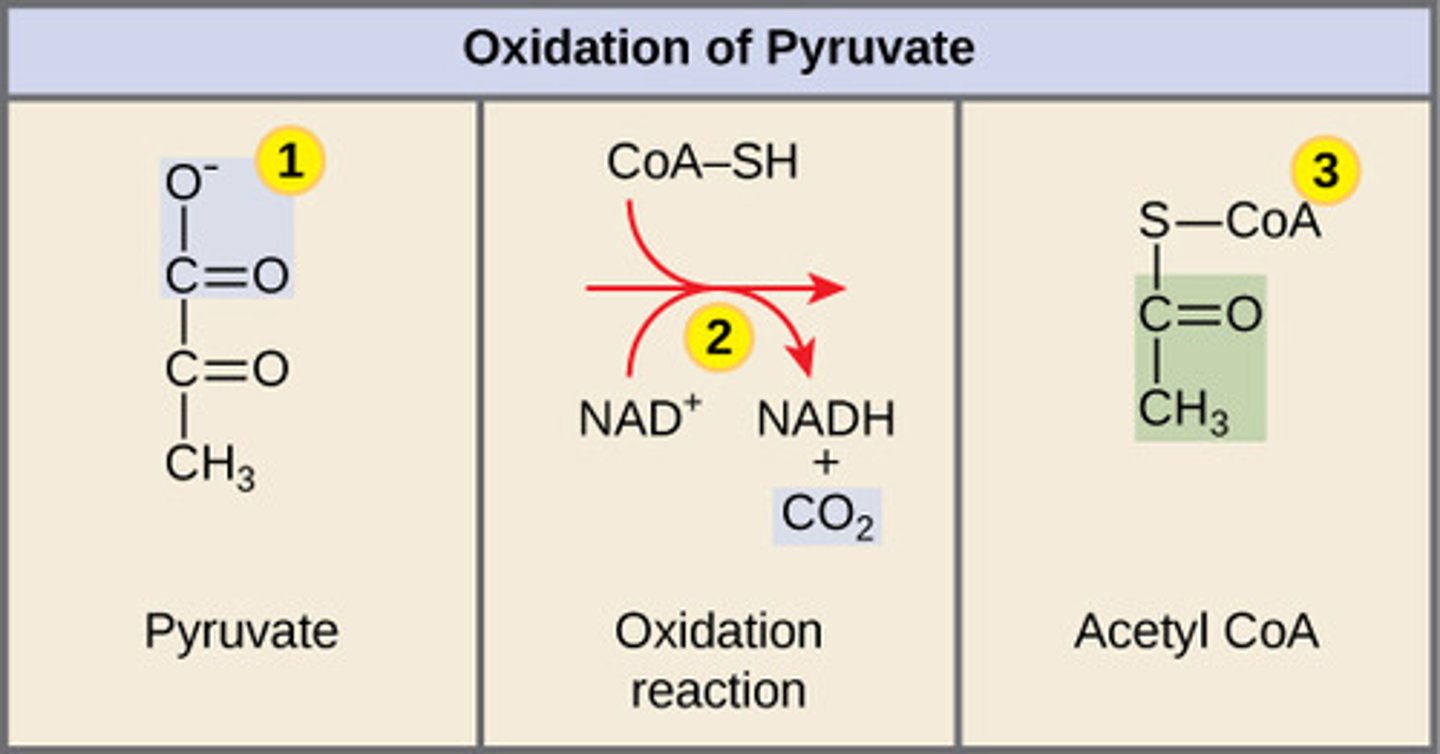
By which enzyme is pyruvate decarboxylation catalyzed?
pyruvate dehydrogenase
complex (PDC)
Where does the citric acid cycle (CAC) take place?
mitochondrial matrix
What is the first step of the CAC?
acetyl CoA merges
with oxaloacetate
to form citrate
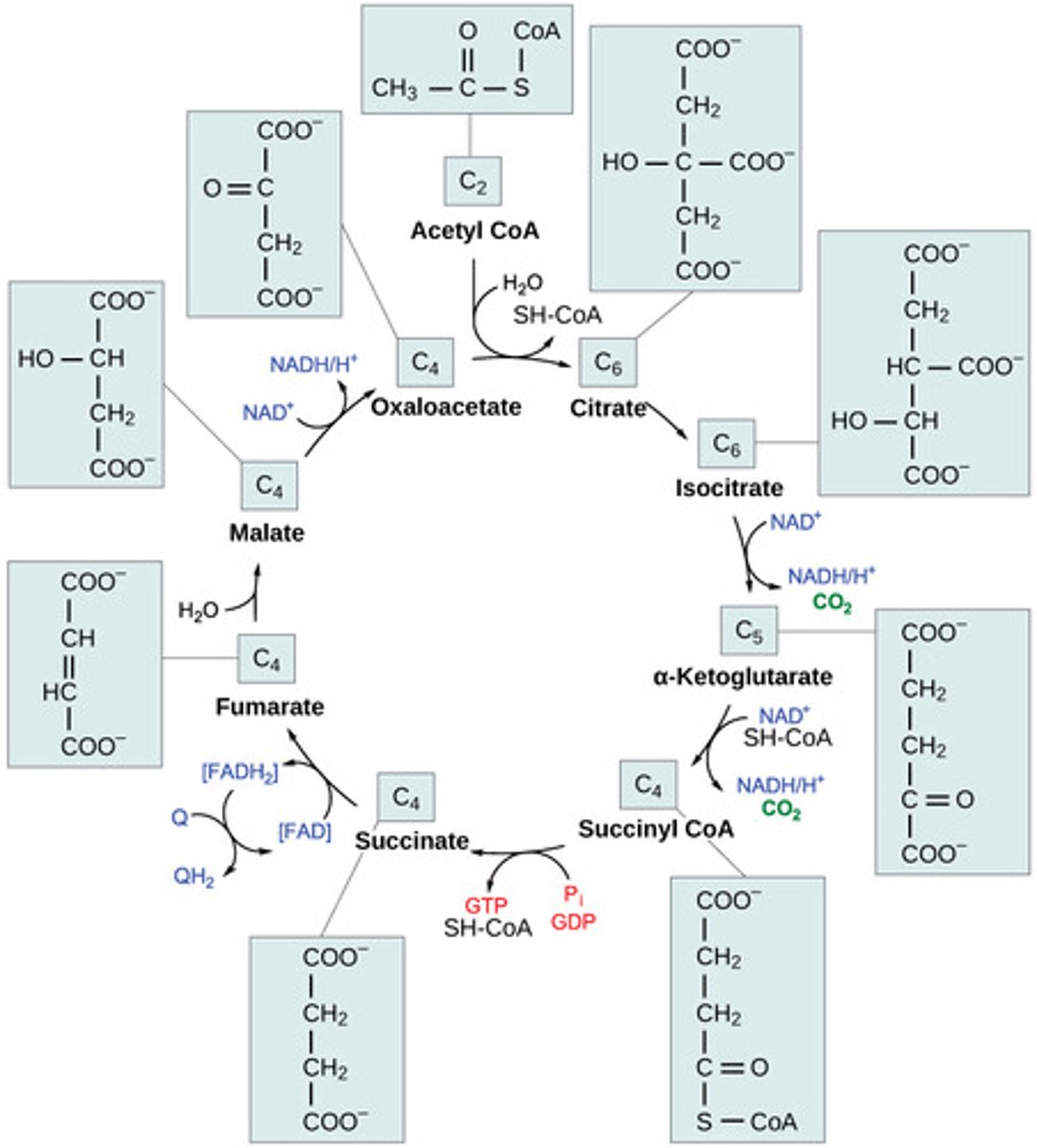
In the CAC, what are the products for every molecule of glucose entered?
6 NADH + 2 FADH2 + 2 GTP + 4 CO2
(Note: 2 pyruvate molecules
per glucose molecule)
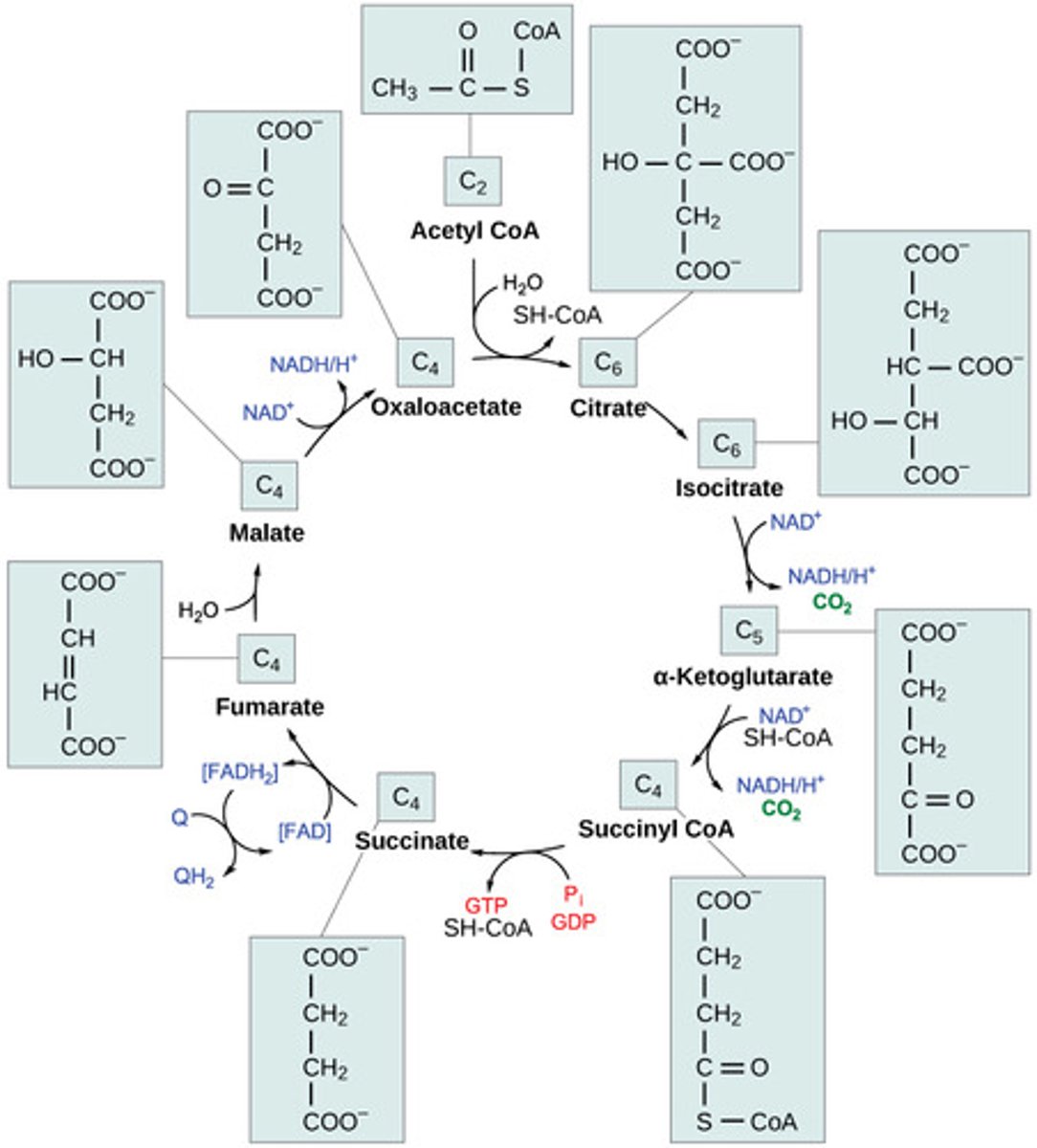
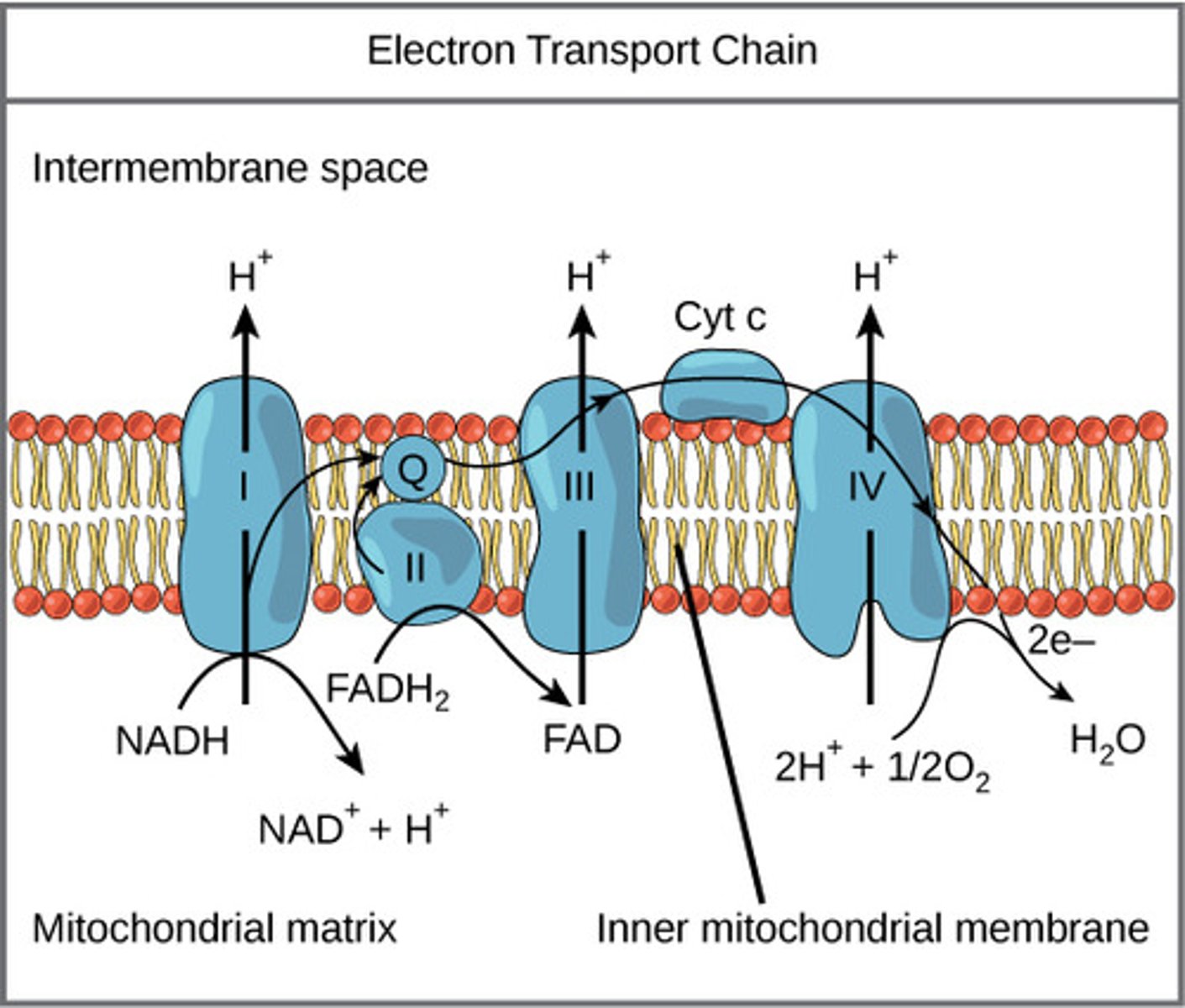
Where does the electron transport chain (ETC) take place?
mitochondrial inner membrane
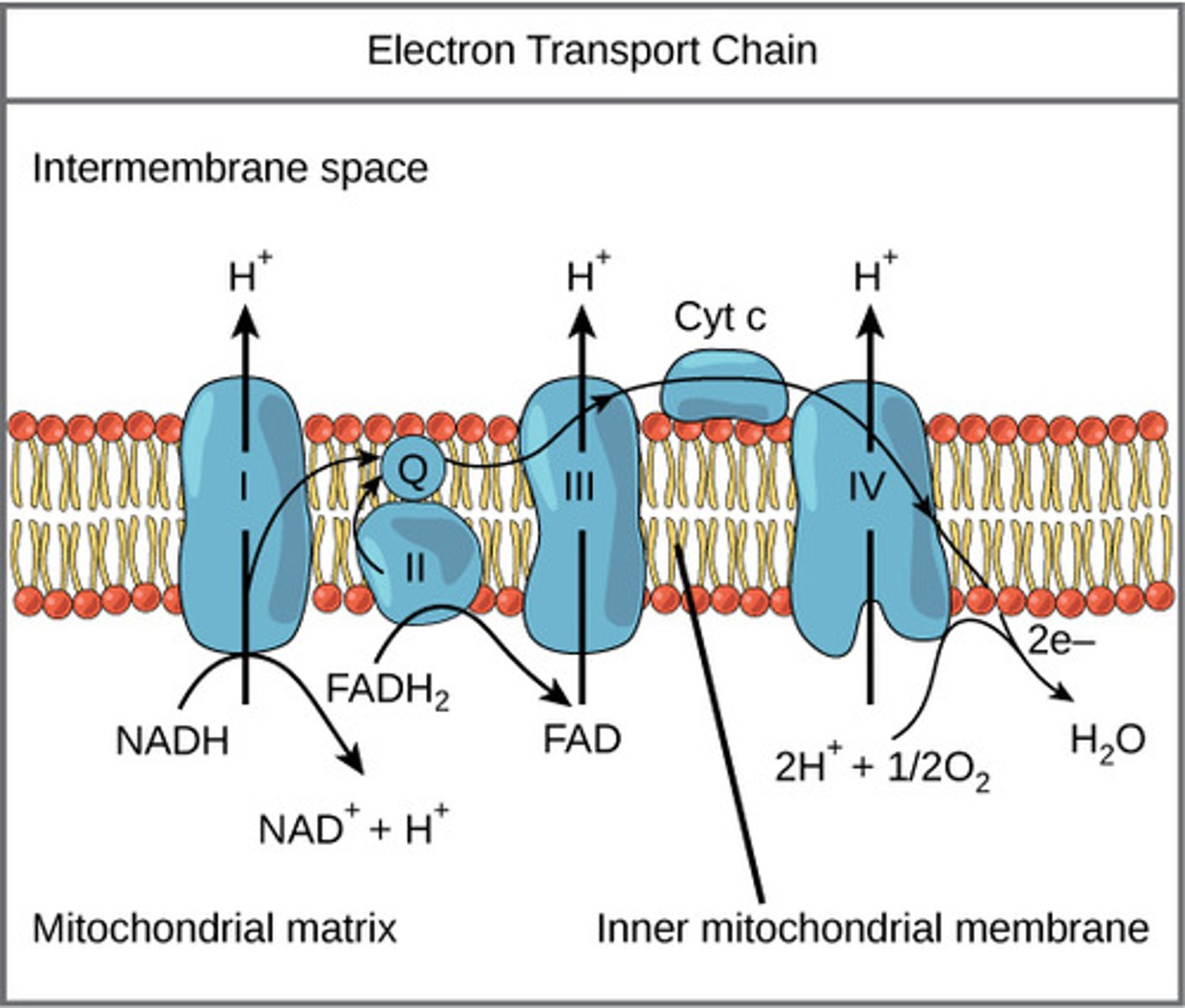
What is the overall reaction of oxidative phosphorylation?
ADP → ATP from NADH and FADH2
How does NADH compare to FADH2 in terms of energy production?
NADH makes more energy than FADH2 (3:2 ratio)
What molecule is the final electron acceptor of the ETC?
oxygen
(Note: combines with H+ protons that cross to make water)
What source supplies the energy for synthesizing ATP?
proton gradient
(note: the whole
point of the ETC
is to establish a
proton gradient
to power ATP synthase)
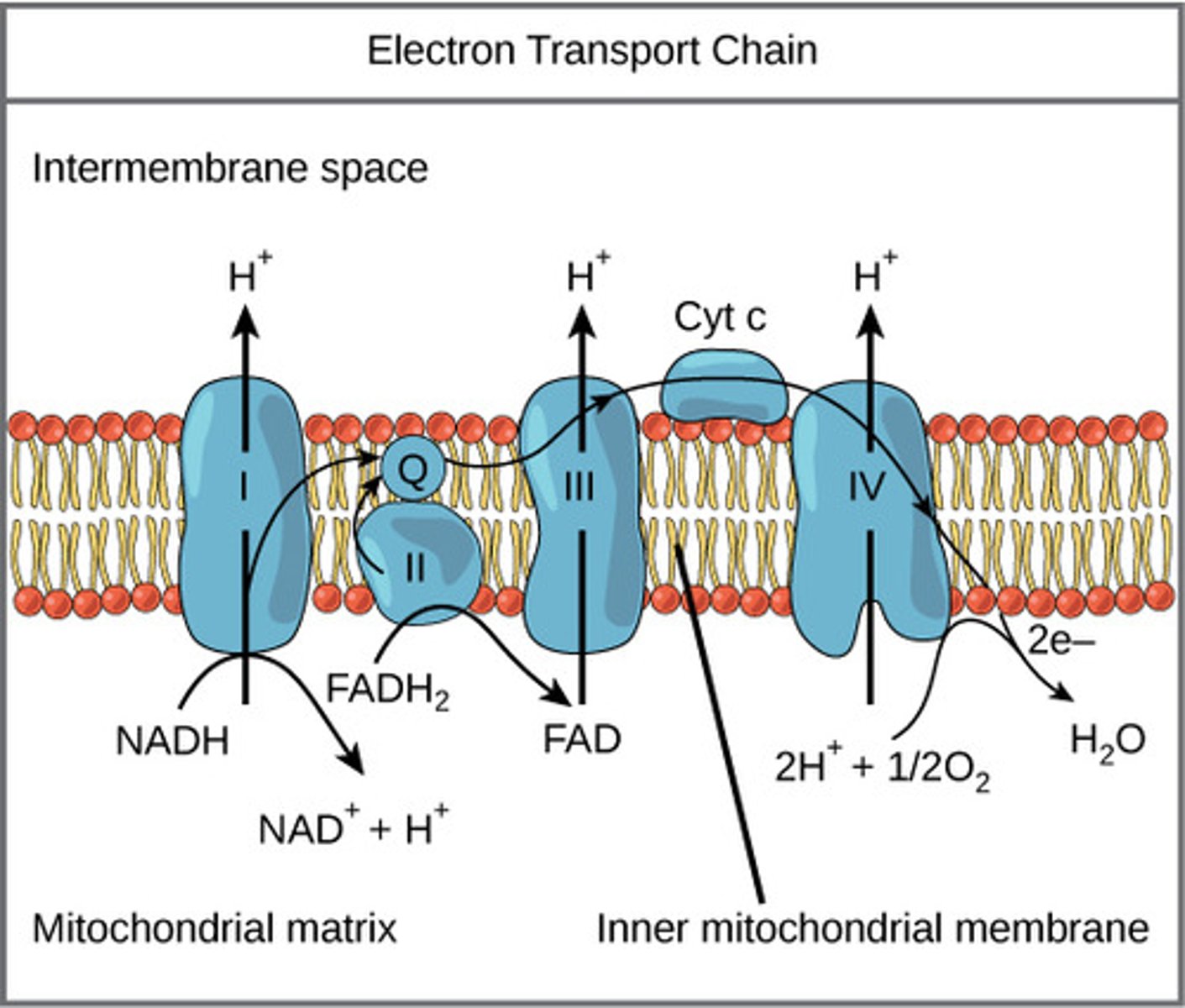
As the electron carriers take electrons from NADH and FADH2, what are they pumping into the intermembrane space?
protons
(Note: building up the proton gradient)
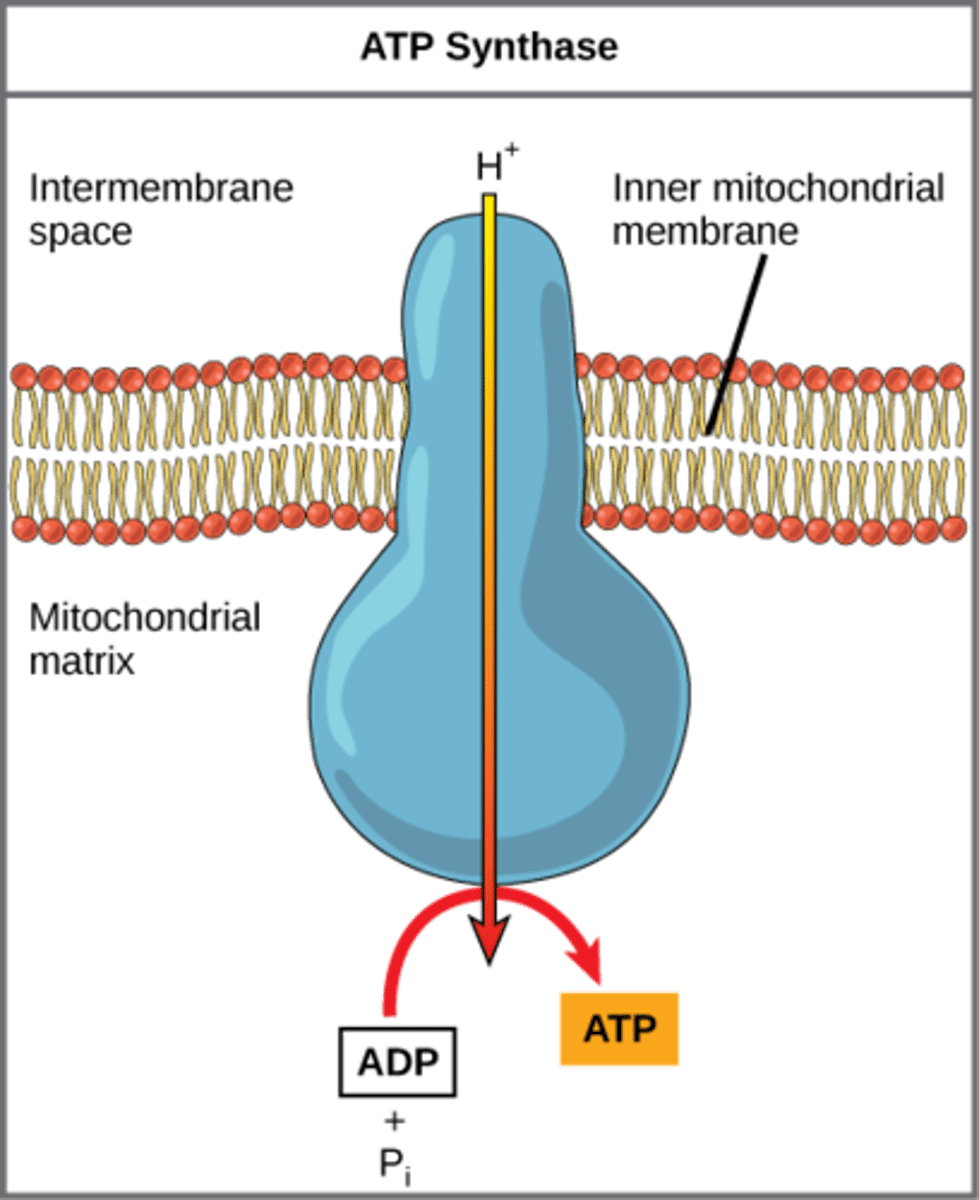
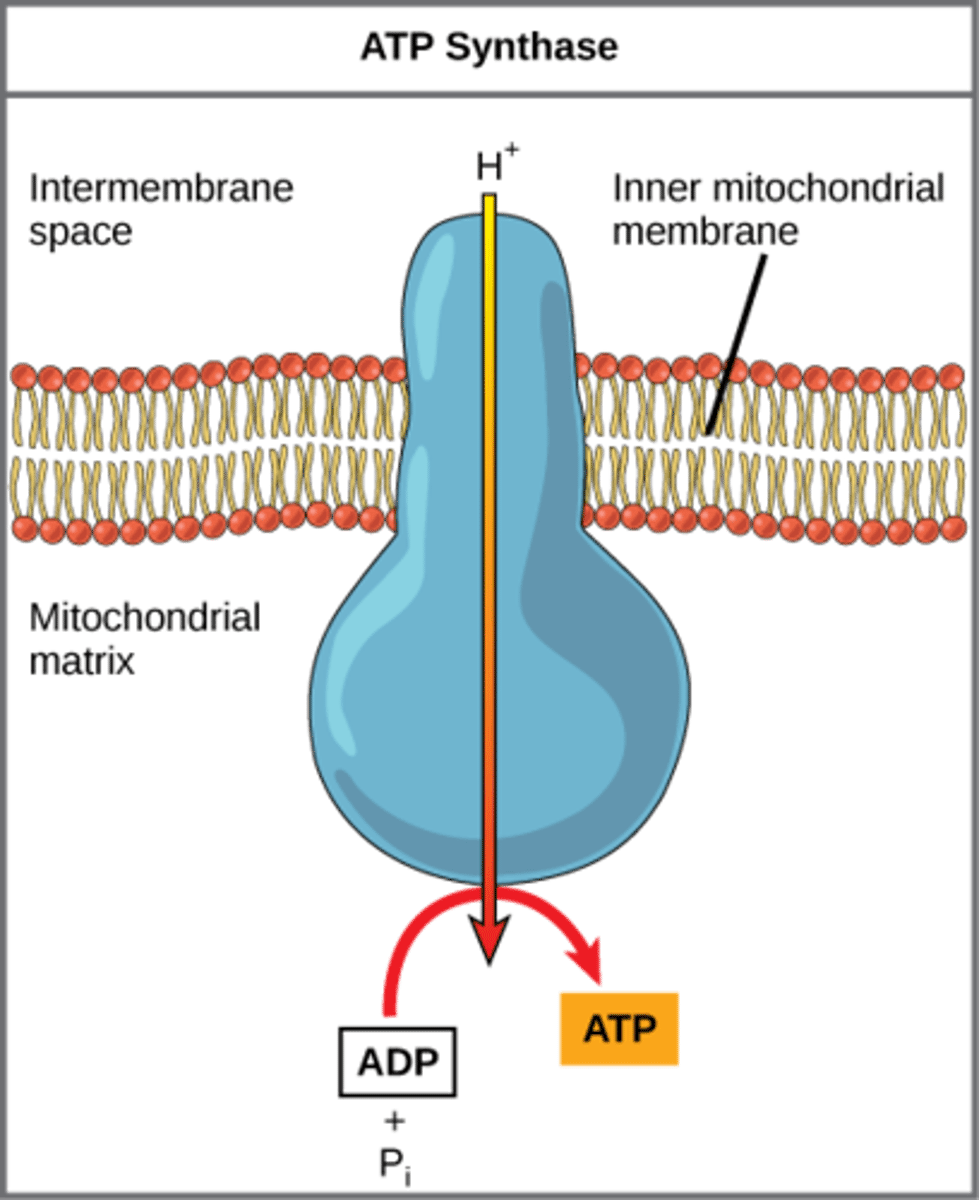
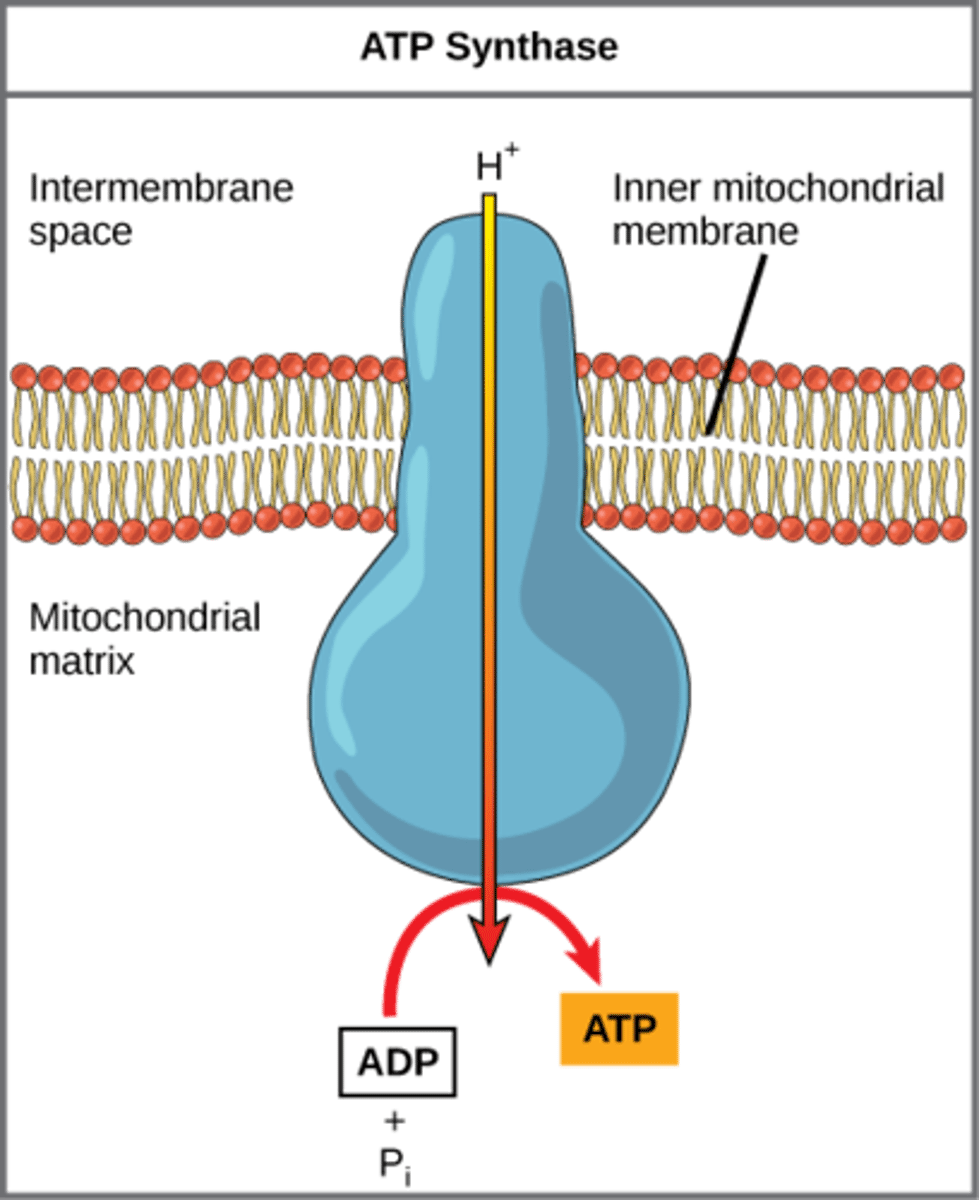
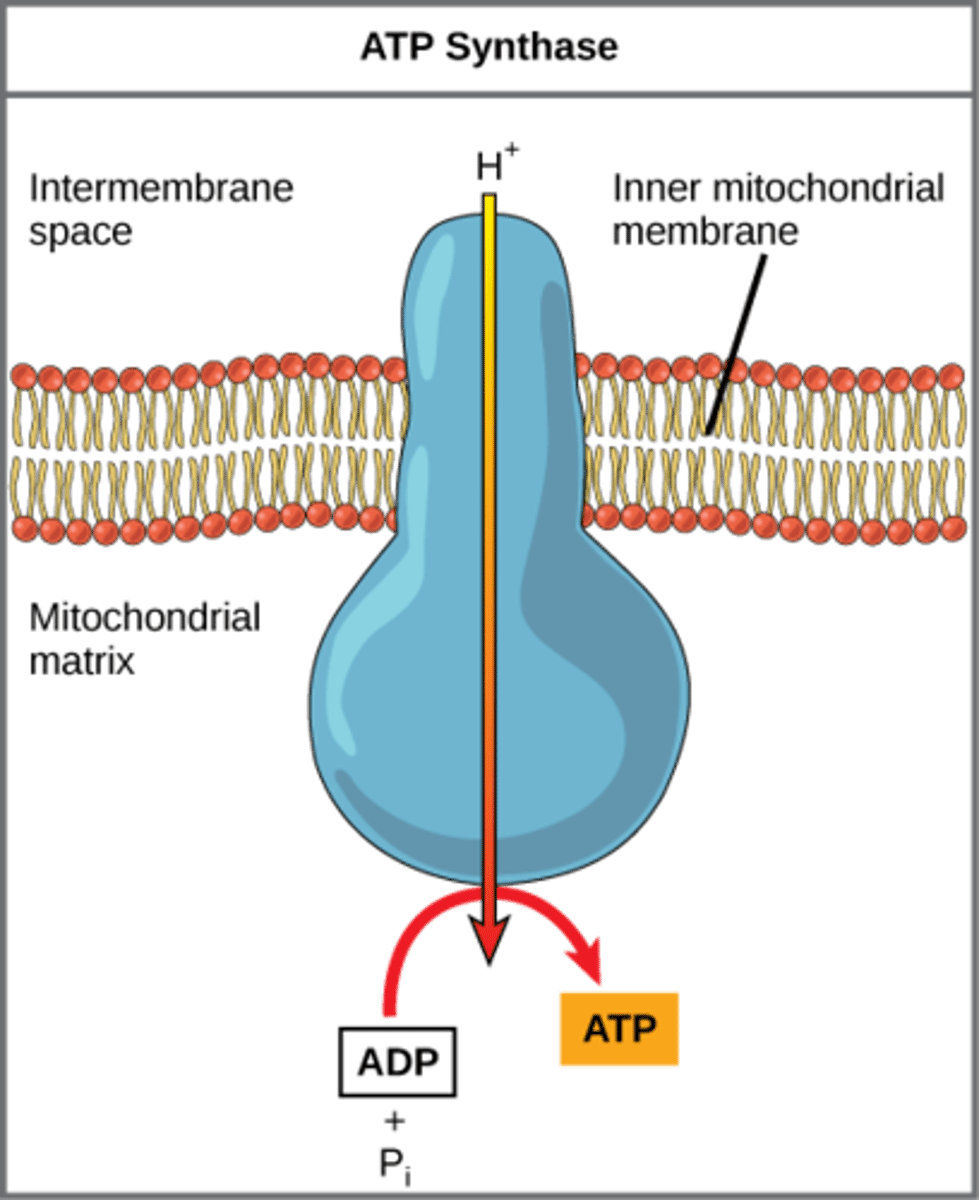
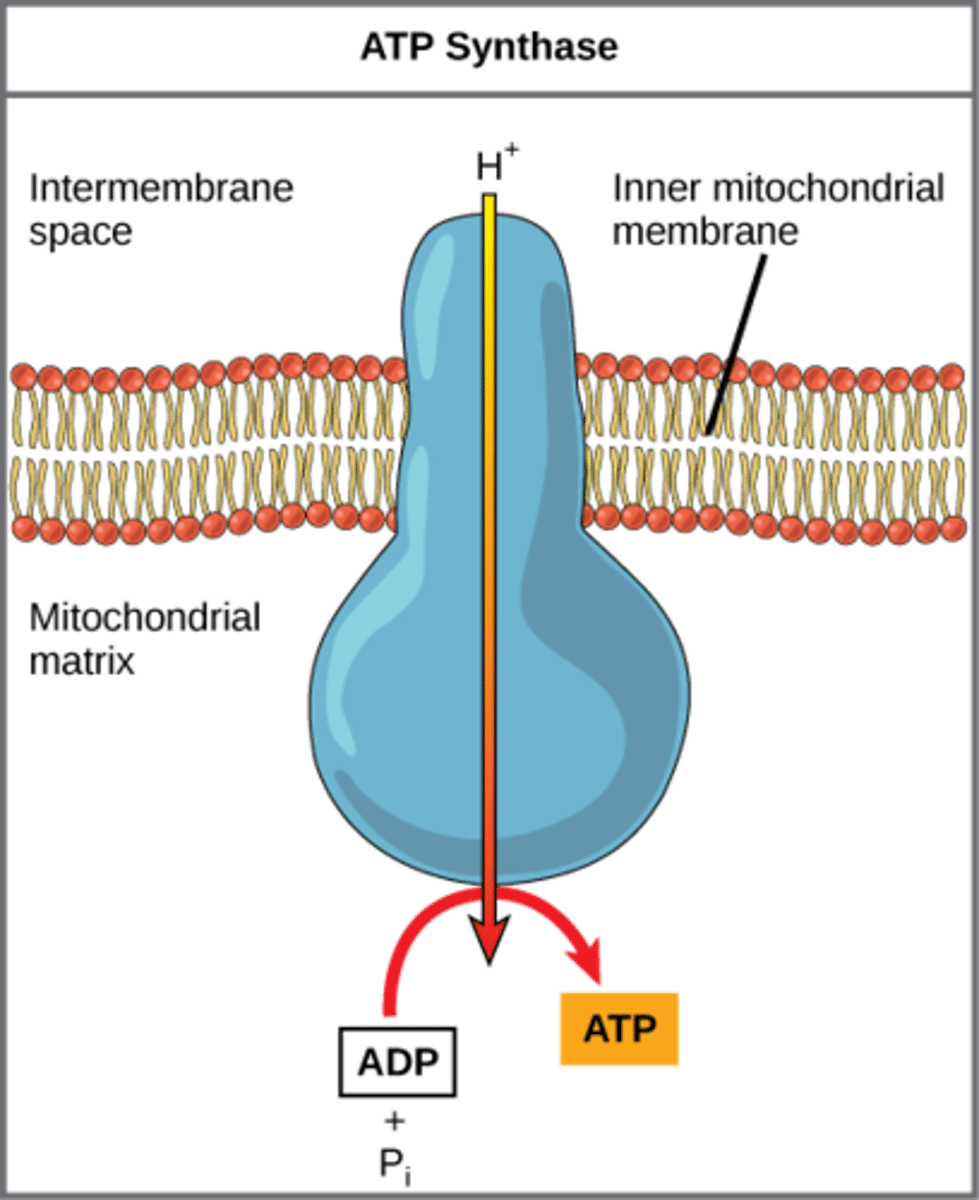
How does ATP synthase make ATP?
add phosphate to
ADP through using
H+ proton gradient
What is a soluble carrier dissolved in the membrane that can be fully reduced/ oxidized as it passes electrons?
coenzyme Q (CoQ) / ubiquinone
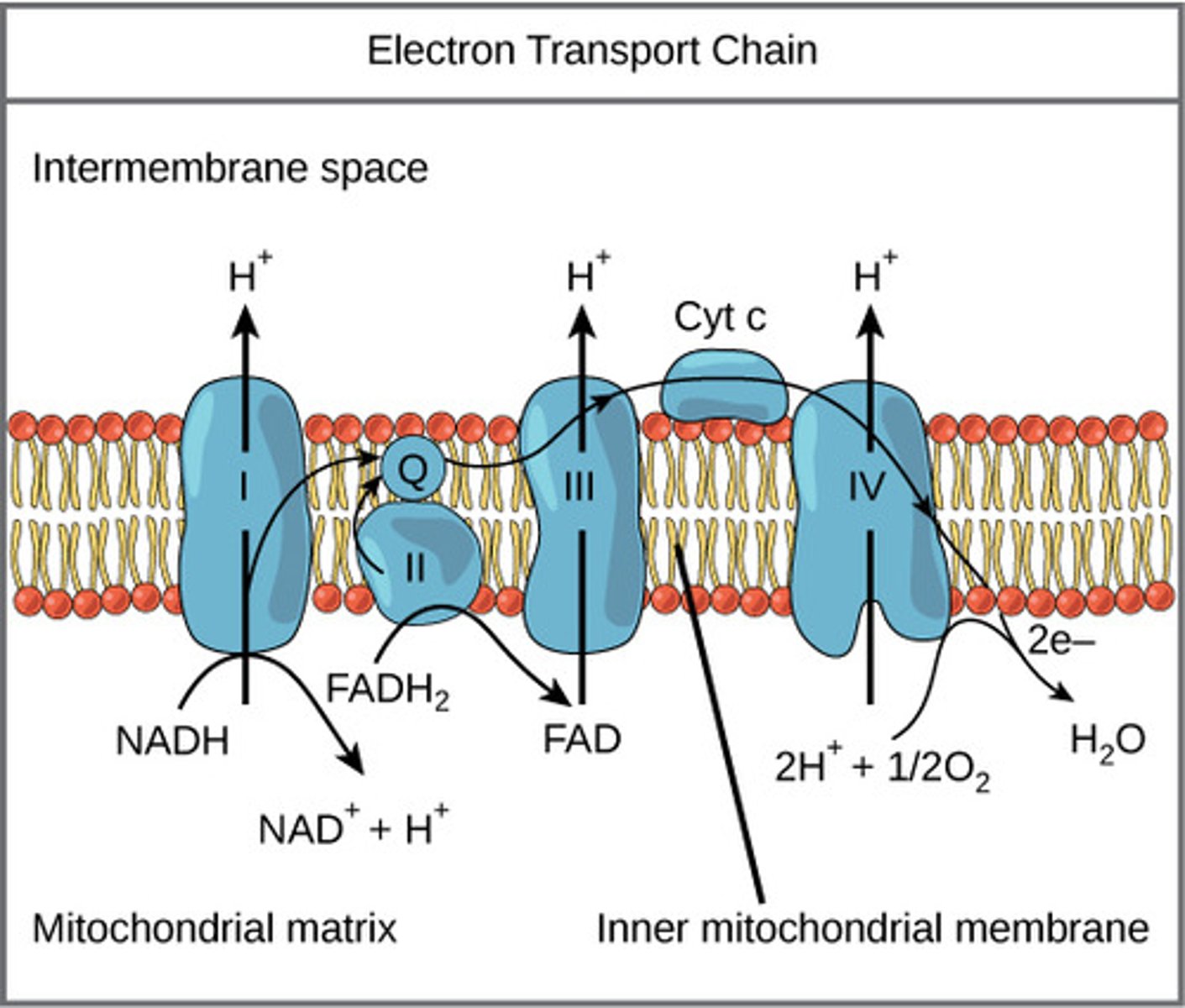
What is a protein carrier in the ETC, is common in many living organisms, and is used for genetic relations?
cytochrome C
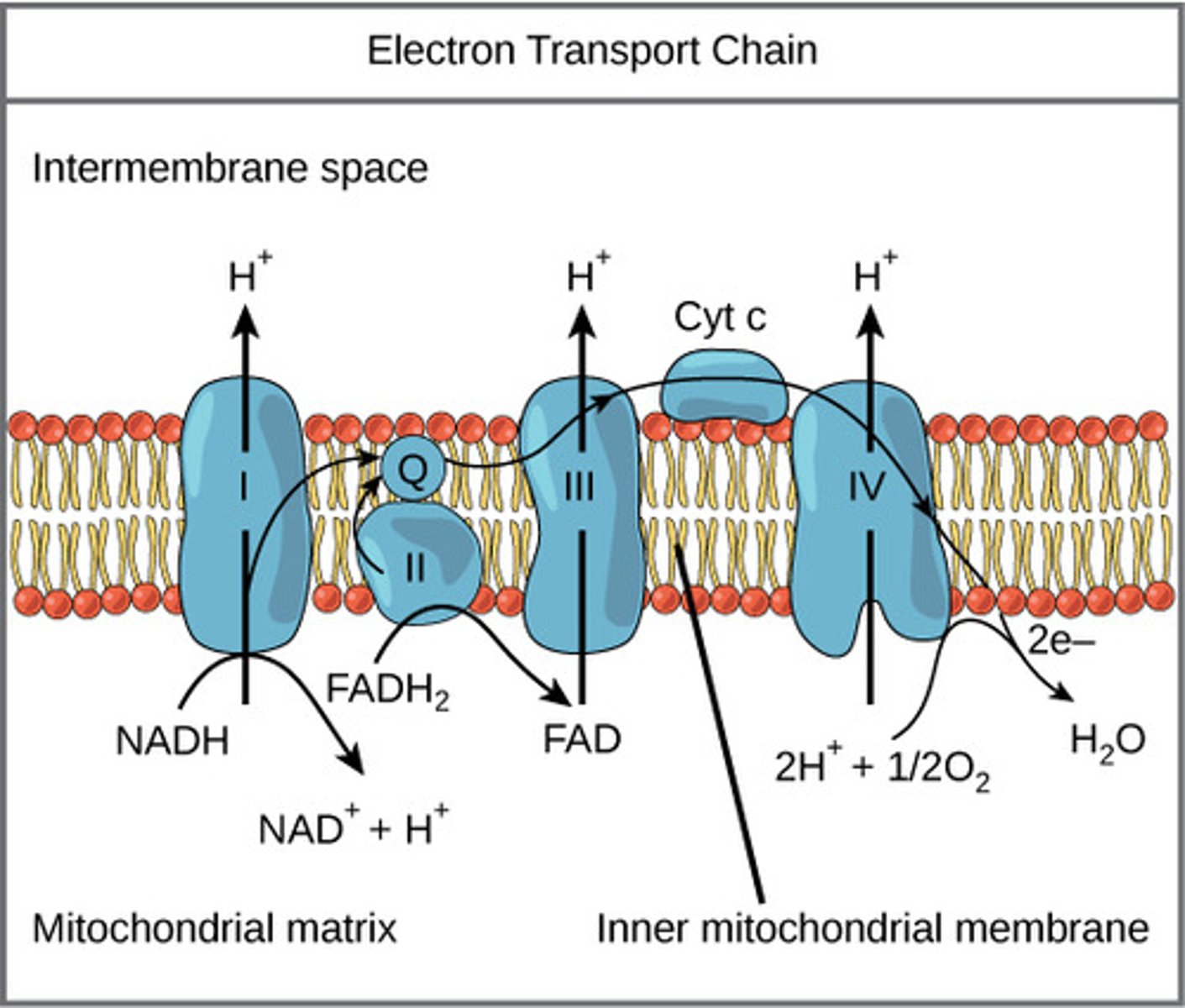
What special substances do cytochromes have that donate or accept electrons for redox reactions?
non-protein elements like iron
How much ATP comes from 1 molecule of glucose during cellular respiration in eukaryotes?
36 ATP
How much ATP comes from 1 molecule of glucose during cellular respiration in prokaryotes?
38 ATP
(Note: no mitochondria - so more efficient transfer of energy)
What is the mechanism of ATP generation that occurs when energy is stored in the form of a proton concentration gradient?
chemiosmosis
What reaction do NADH and FADH2 undergo to transfer proteins across the mitochondrial membrane?
oxidation
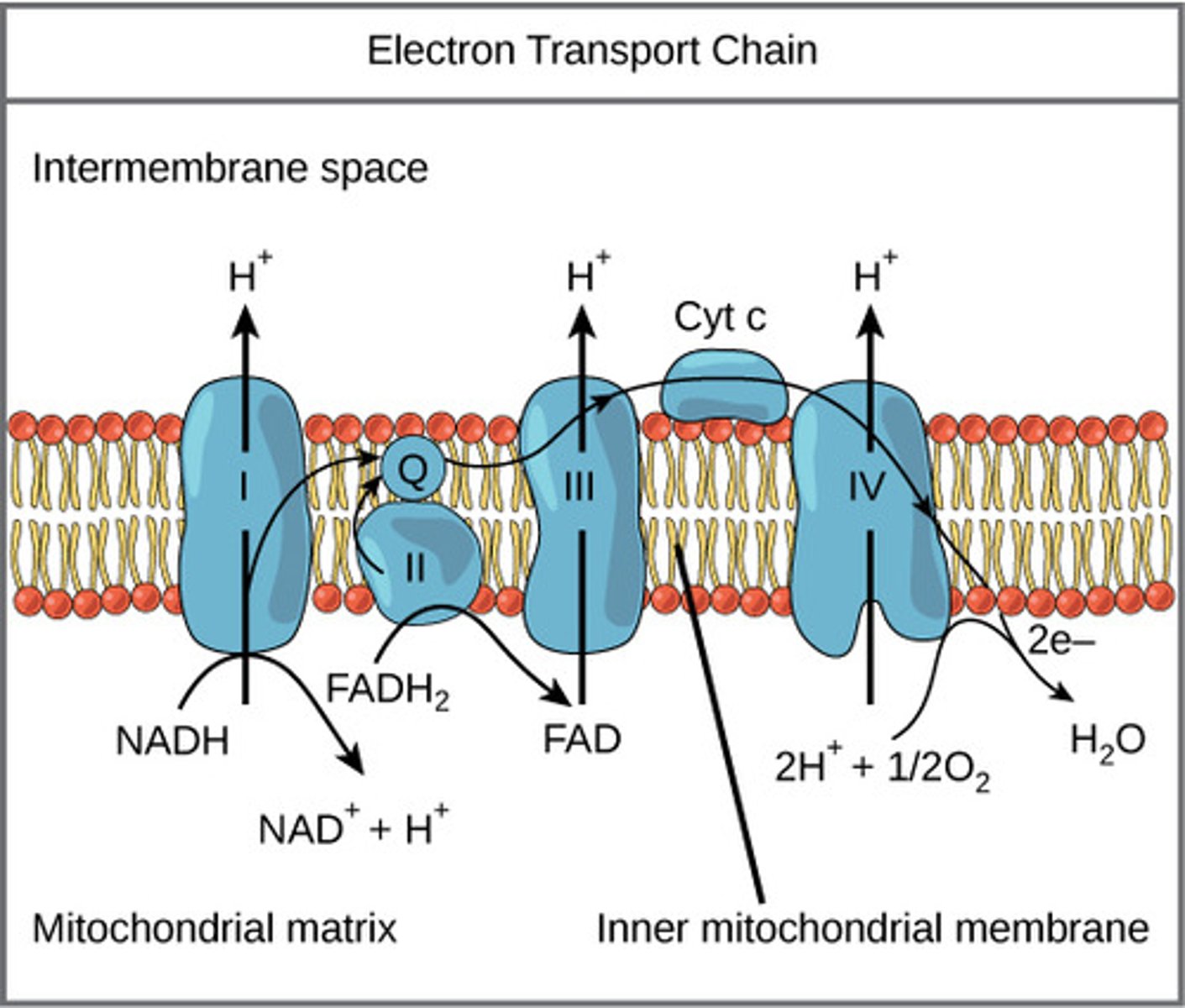
What is the force of the protons flowing through ATP synthase called?
proton motive force
What type of gradient is created through the proton pumping process in the ETC?
pH and electrical
What does higher proton concentration mean for pH?
lower pH
Why is ATP unstable?
3 negative phosphate groups
repel one another
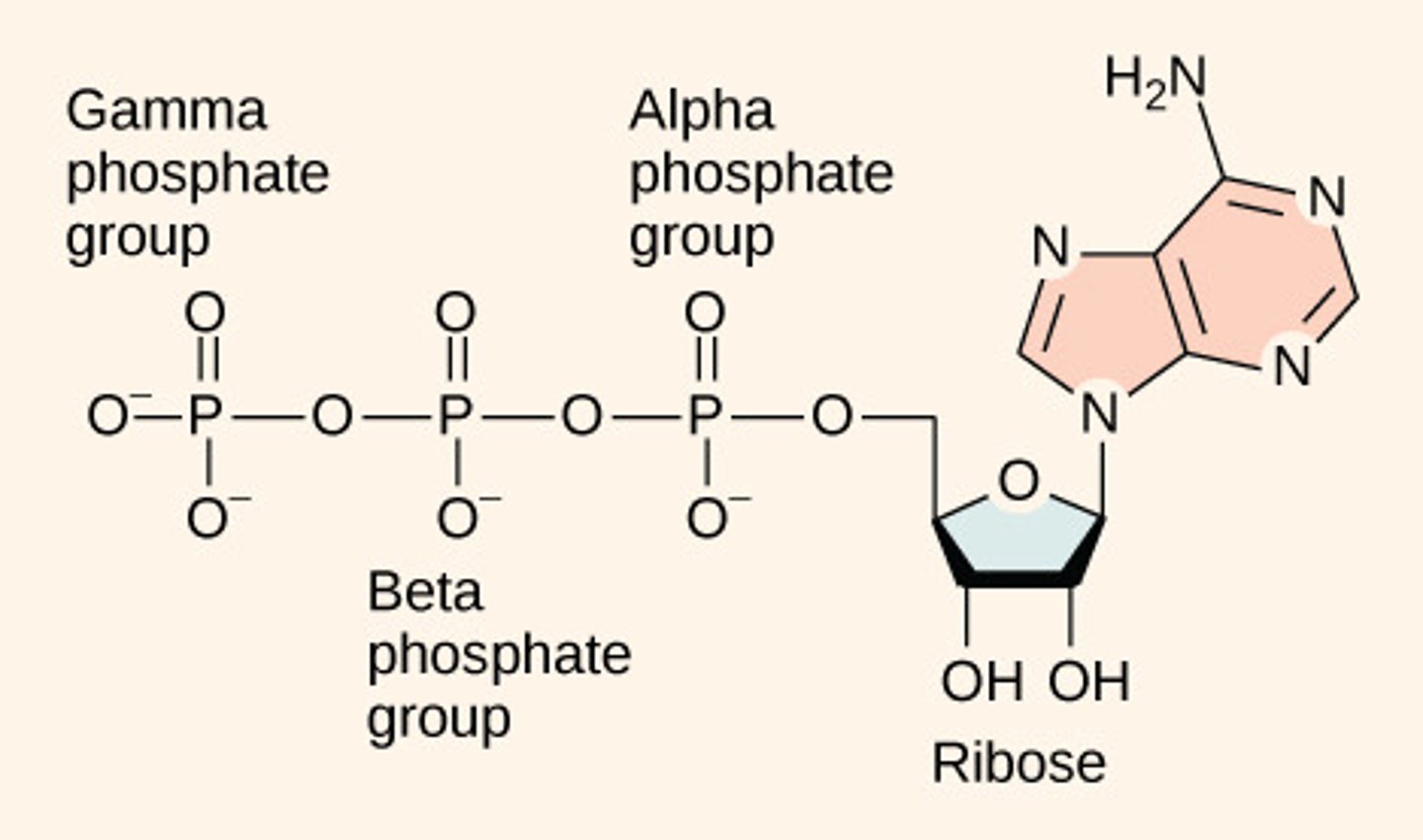
Why does hydrolysis of ATP release energy?
creates a more stable
ADP molecule
(Note: transitions from
less stable to more
stable molecules
always releases energy)
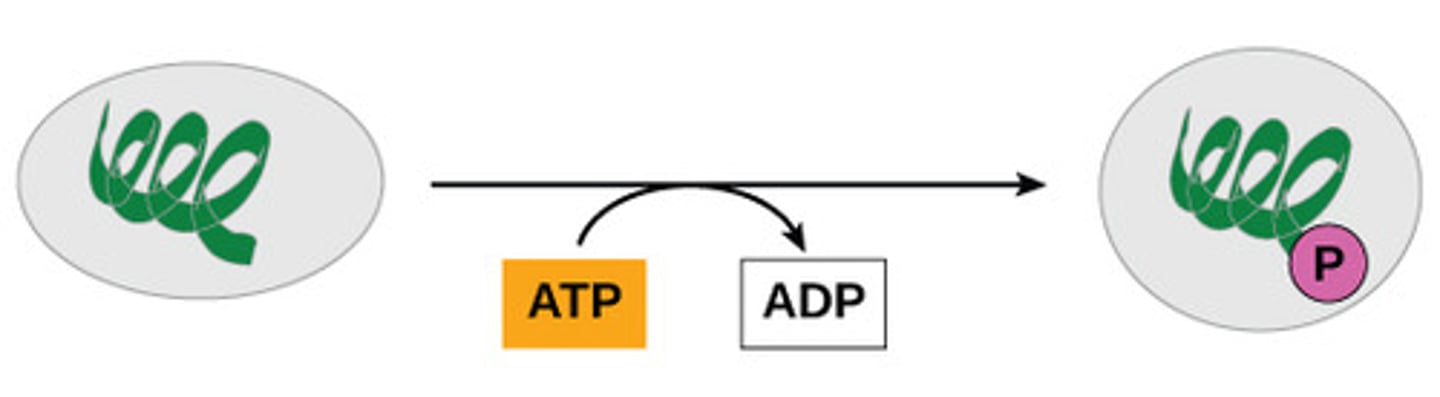
How does ATP provide energy for all cells?
transferring phosphate from
ATP to another molecule
What are the steps involved in anaerobic respiration?
1. glycolysis
2. fermentation
What molecule does fermentation regenerate?
NAD+
(Note: required for the
continuation of glycolysis)
Under normal circumstances (oxygen present), how is NAD+ regenerated?
in the ETC using O2
In what organisms does alcohol fermentation occur?
1. plants
2. fungi
3. bacteria
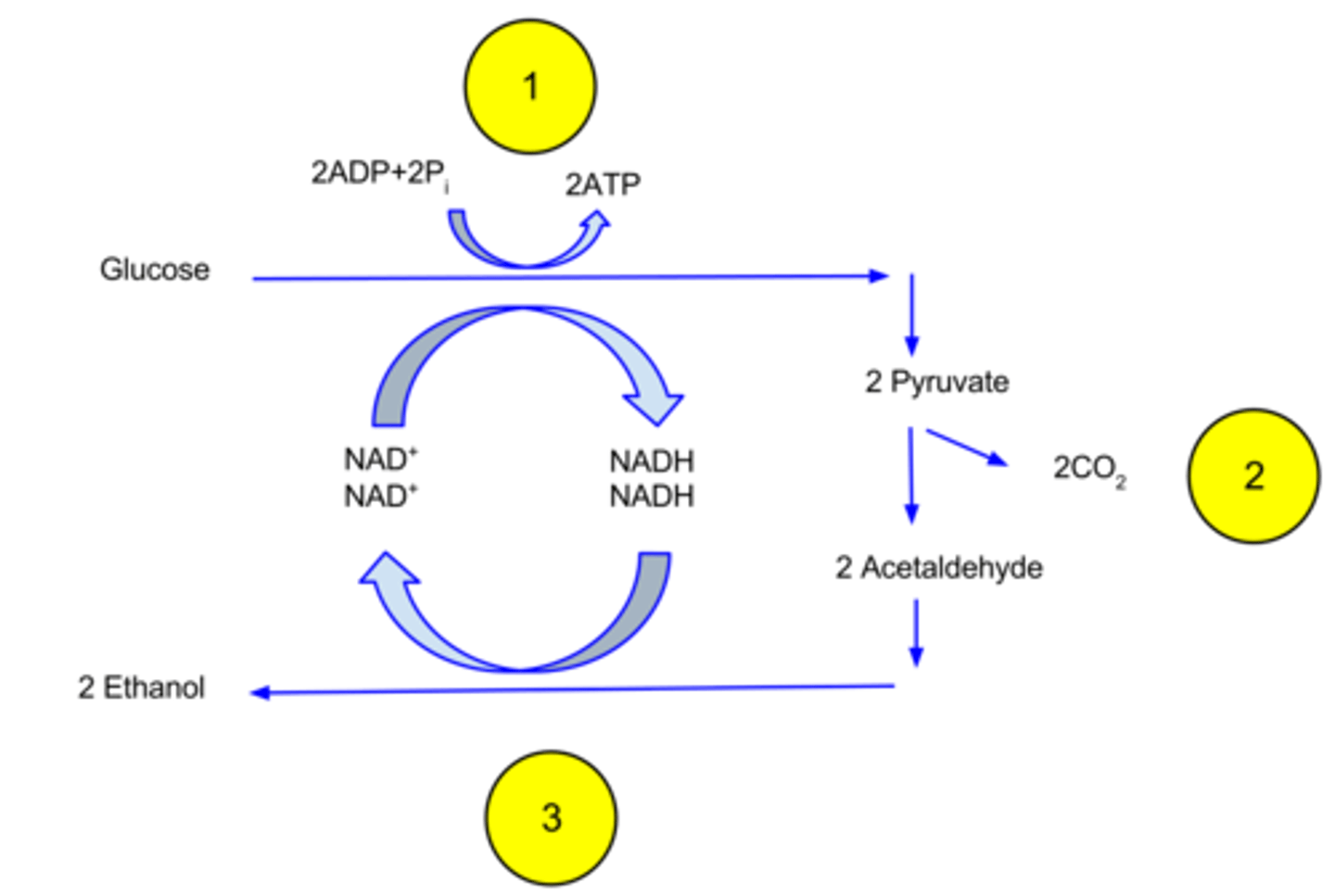
What is the overall process of alcohol fermentation?
1. Pyruvate → acetaldehyde + CO2
2. acetaldehyde → ethanol (and NADH → NAD+)
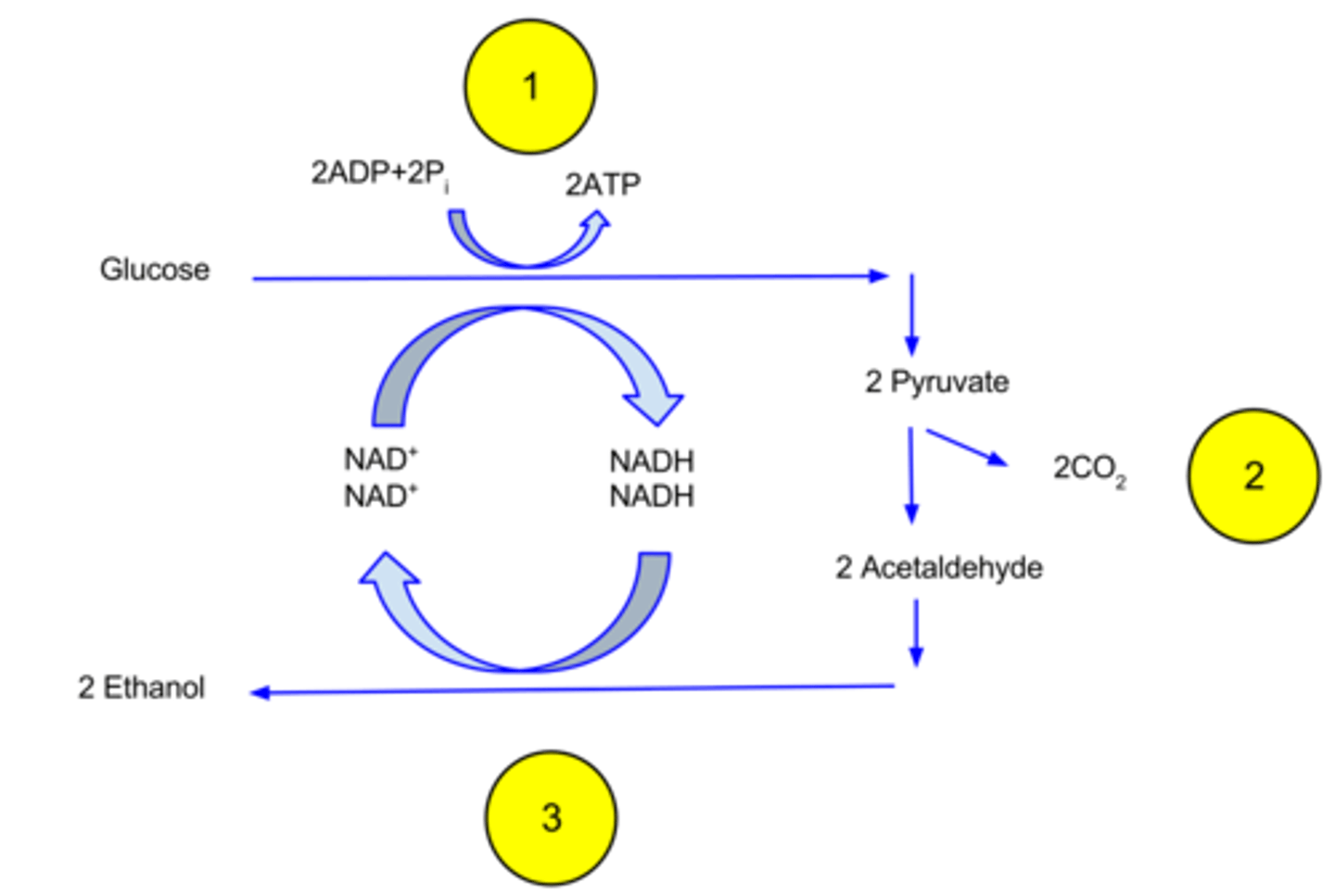
What is the final electron acceptor in alcohol fermentation?
acetaldehyde
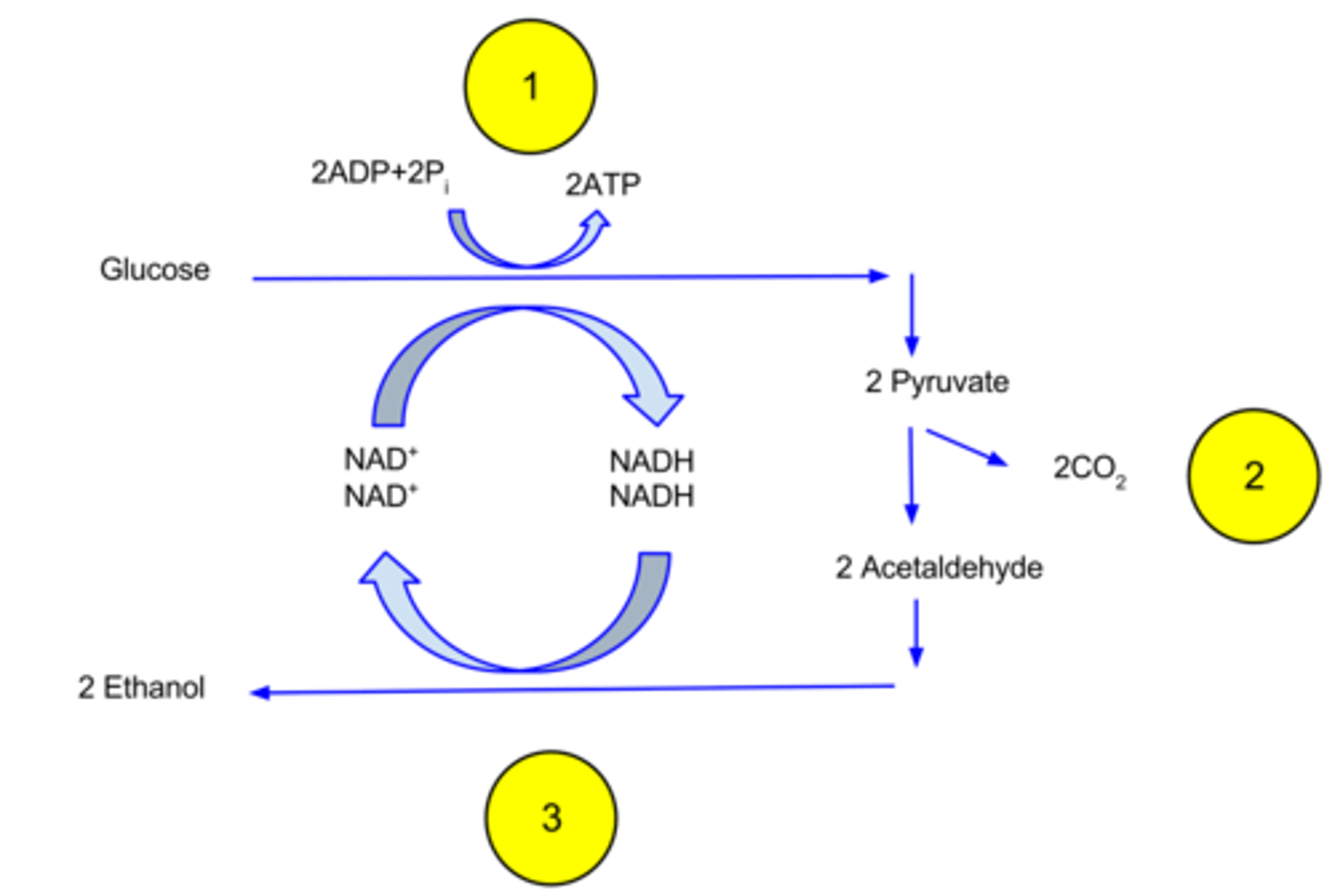
What is the final product of alcohol fermentation?
ethanol
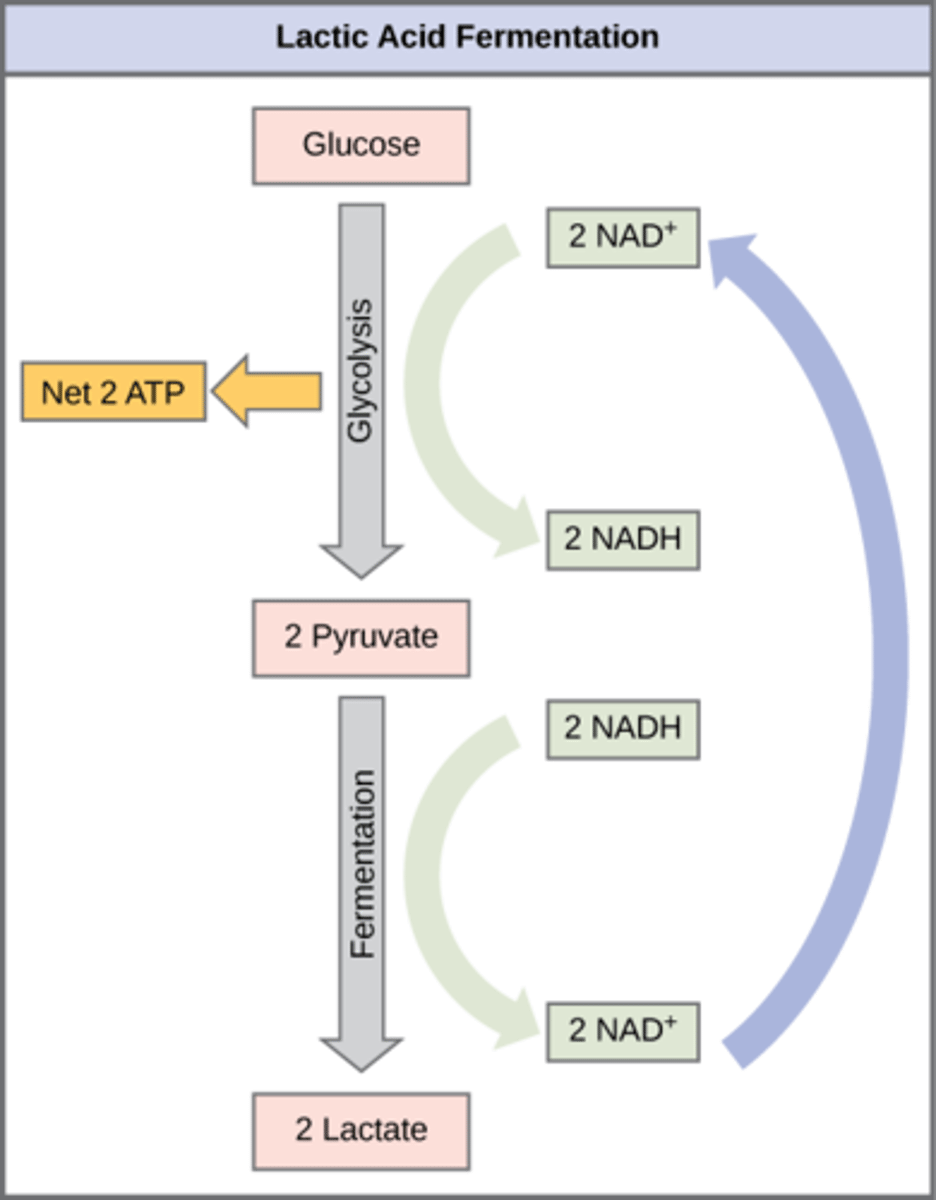
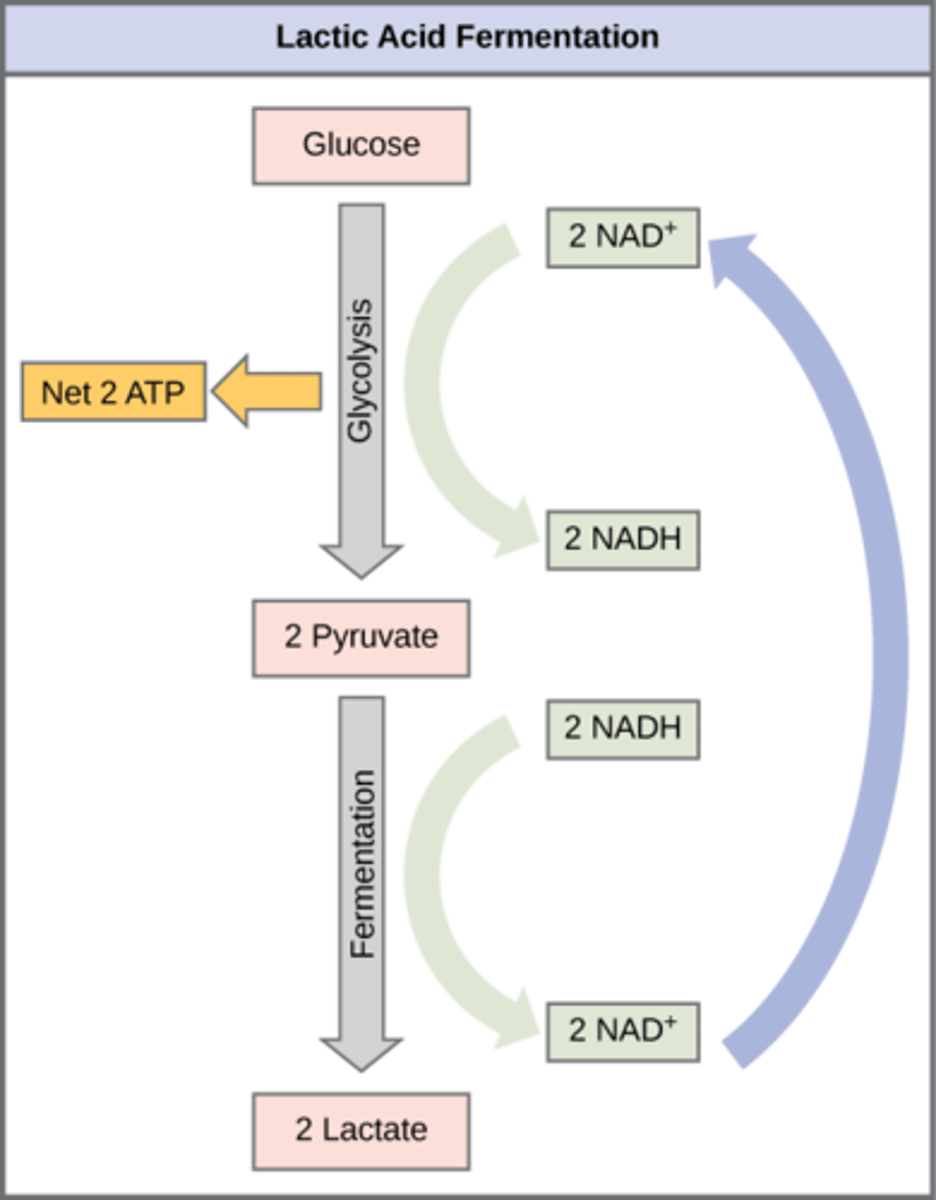
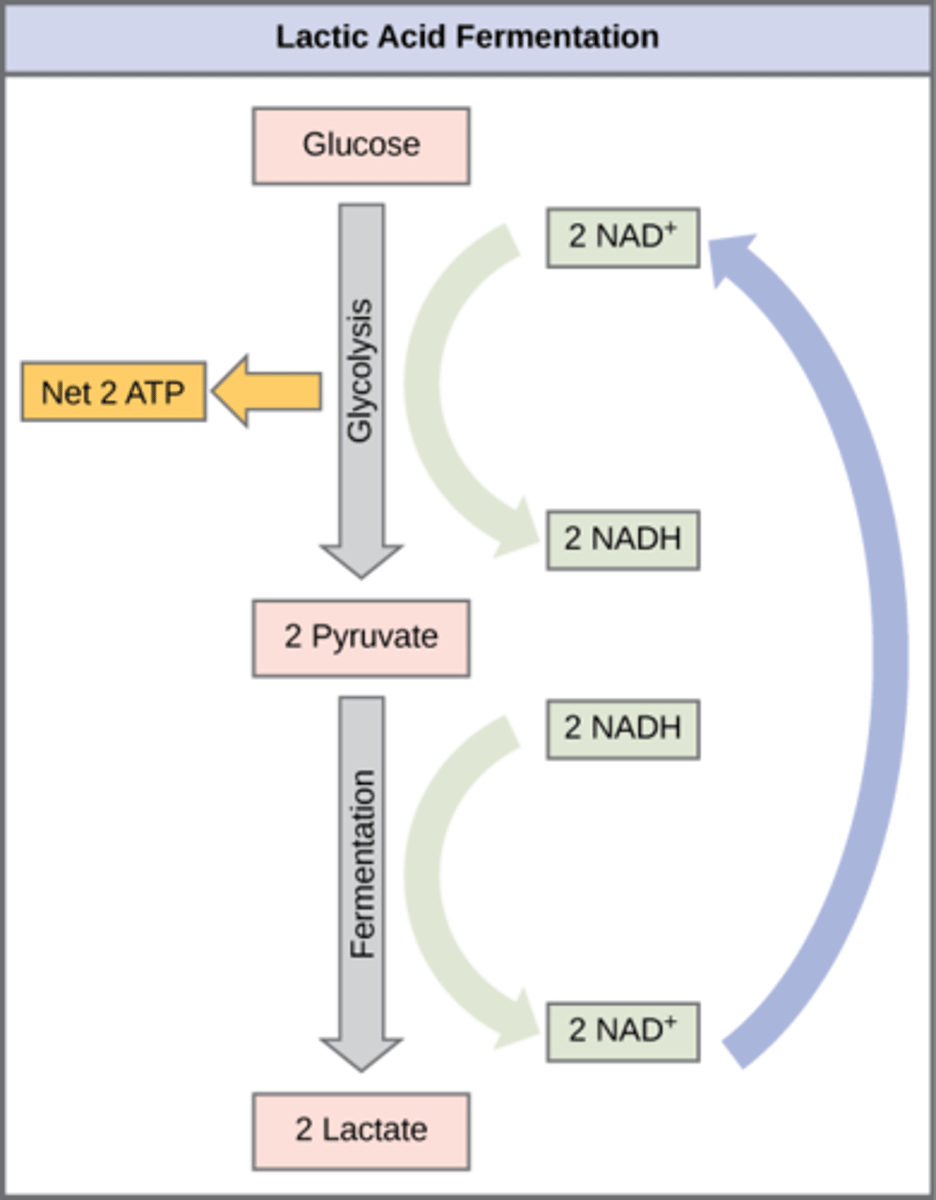
In what organisms does lactic acid fermentation occur?
humans and other microbes
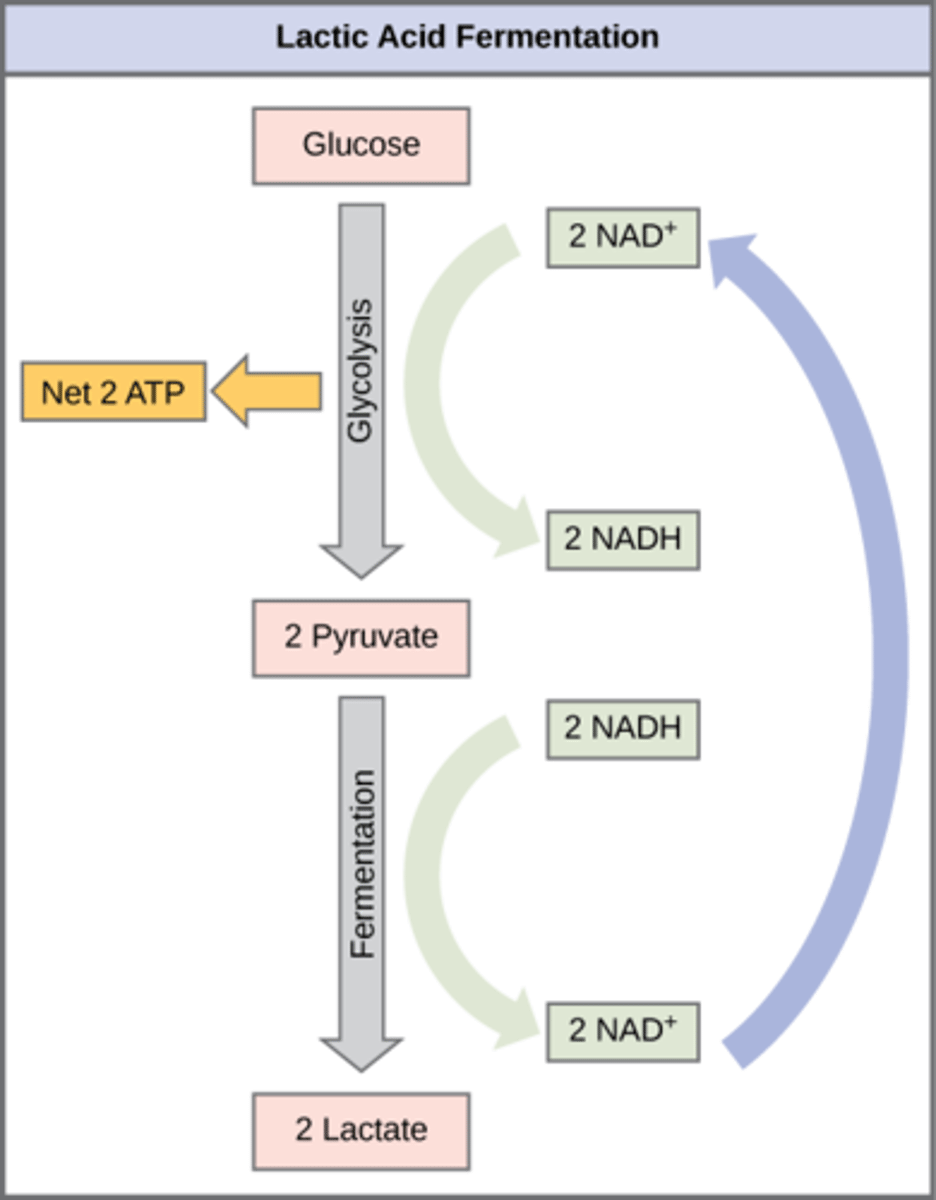
What is the overall process of lactic acid fermentation?
Pyruvate → lactate (and NADH → NAD+)
Where is lactate transported to be converted back into glucose?
liver
(Note: once ATP needs are met)
Which organisms use oxygen when it’s present (more efficient) but switch to fermentation/anaerobic respiration if it isn’t?
facultative anaerobes
Which organisms cannot live in the presence of oxygen?
obligate anaerobes
When glucose is low, in what order does the body use alternative energy sources?
1. other carbohydrates
2. fats
3. proteins
What is another way to obtain glucose other than ingesting food?
gluconeogenesis
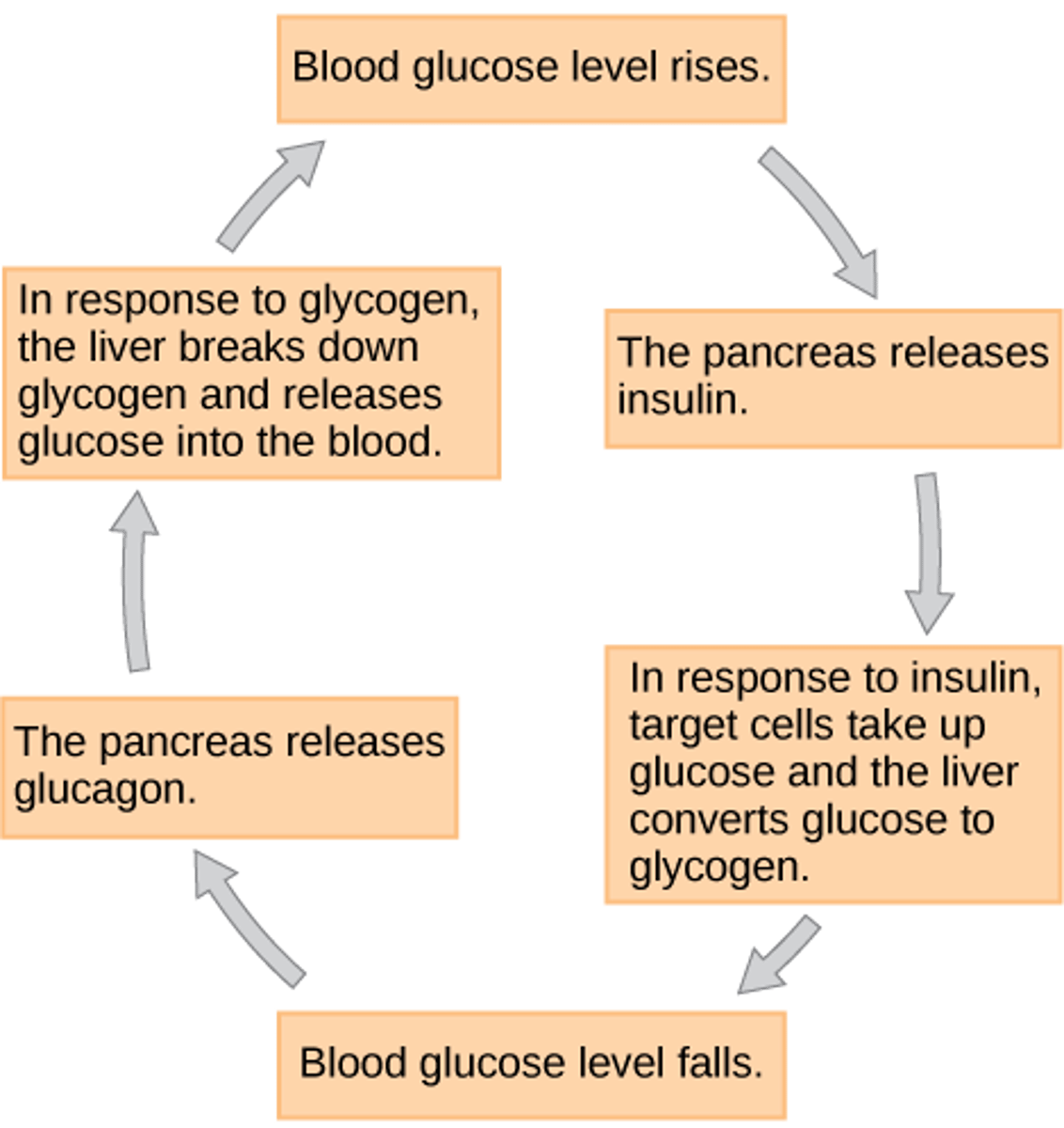
After large meals, into what molecule does insulin prompt the body to convert glucose?
glycogen
(Note: a storage molecule)
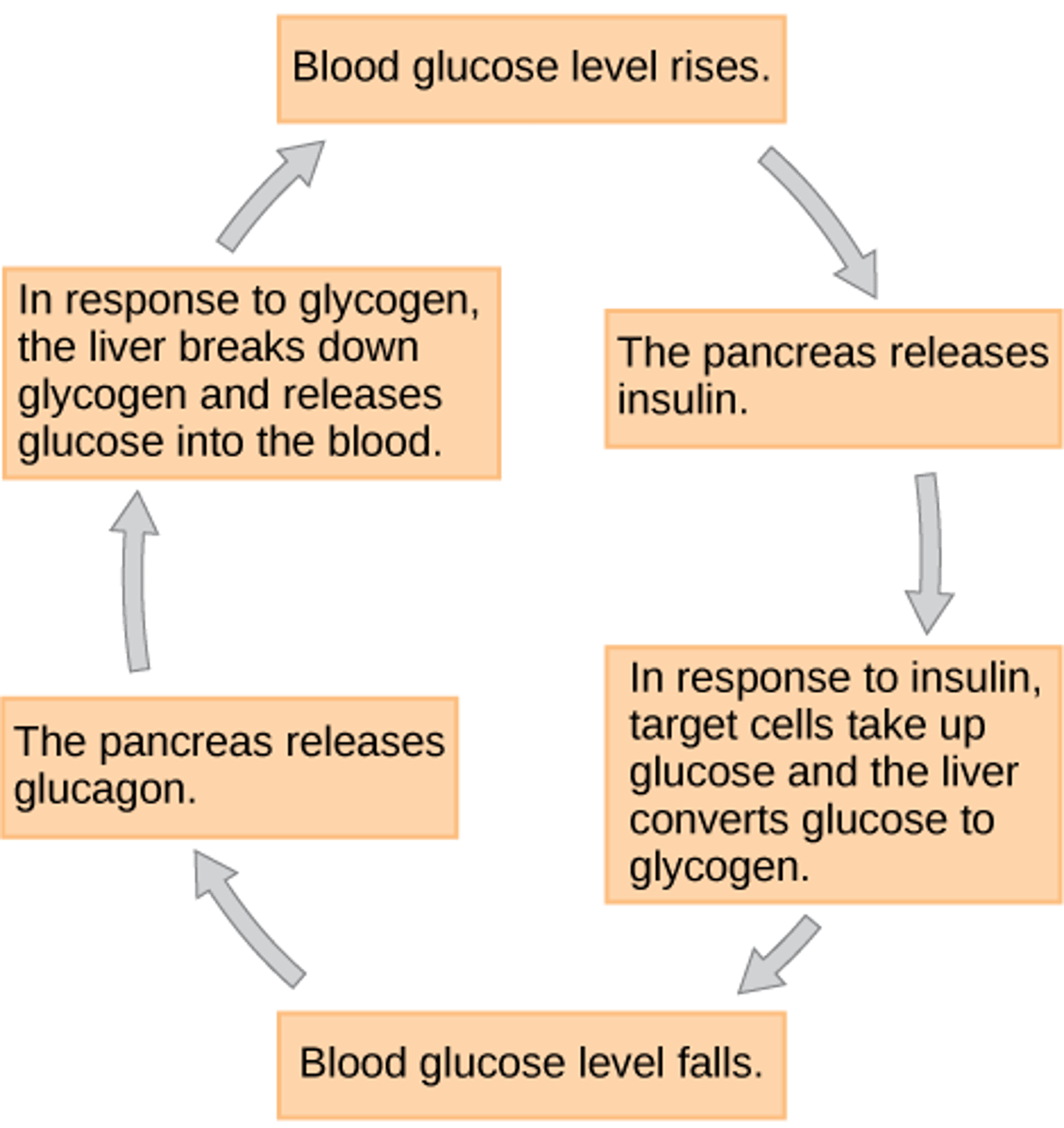
After an extended period of no food, into what molecule does glucagon prompt the body to convert glycogen?
glucose
(Note: mobilize the
stored glycogen for
energy needs)
What effect does insulin have on PFK?
activates PFK
(Note: insulin wants
to use the glucose!)
What effect does glucagon have on PFK?
inhibits PFK
(Note: glucagon wants
to conserve glucose!)
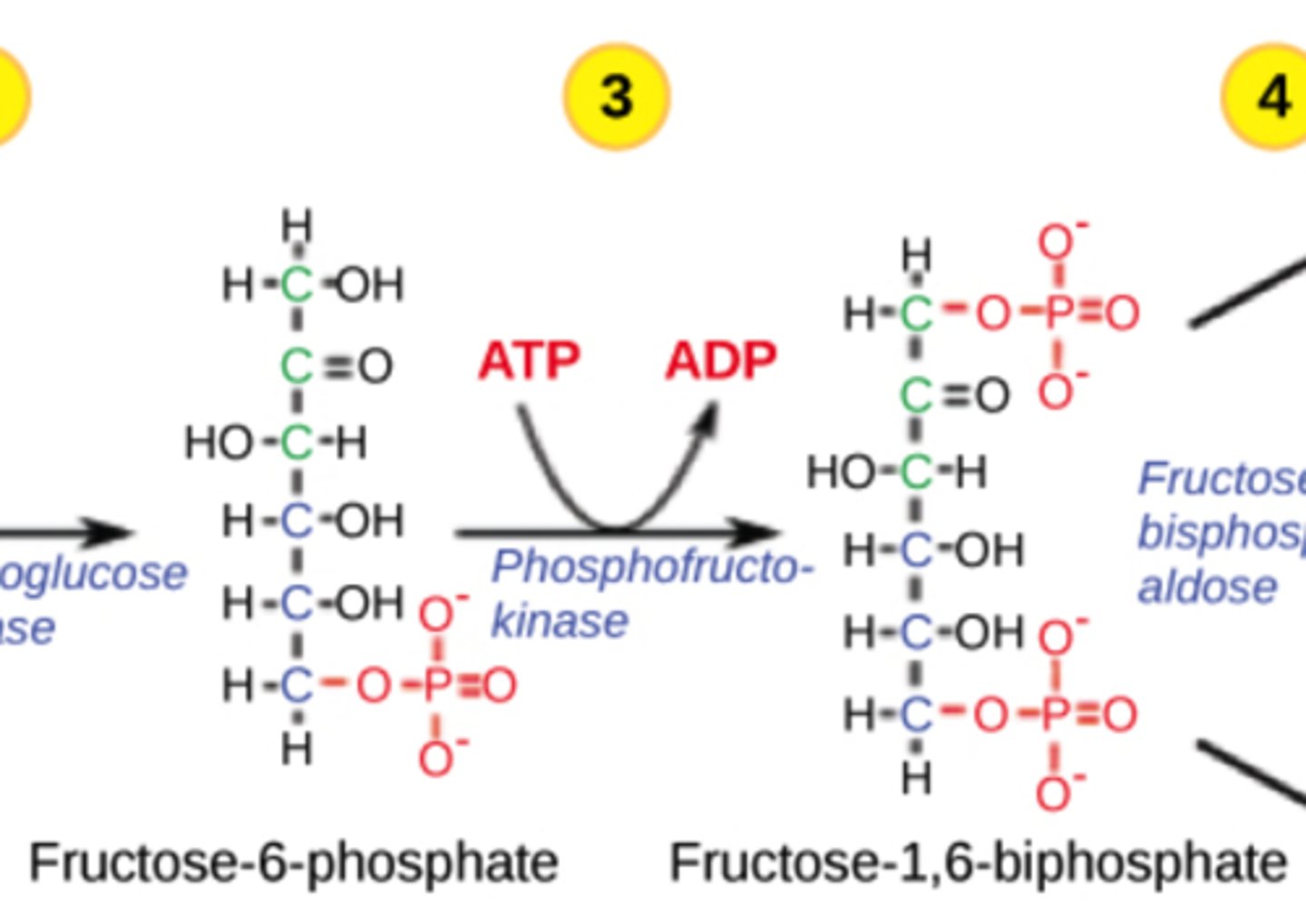
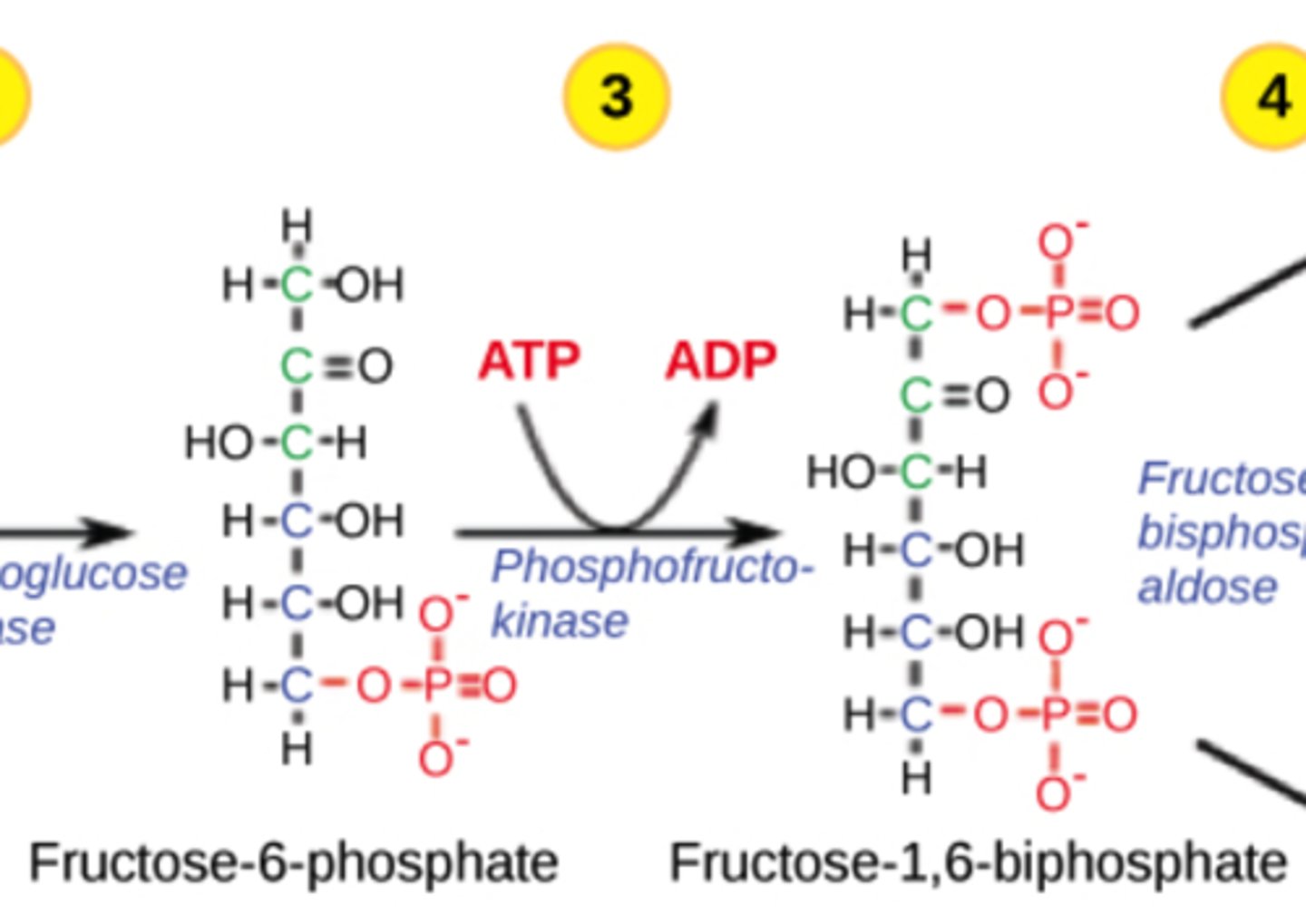
How are disaccharides modified to enter cellular respiration?
hydrolyzed to monosaccharides
What are the cells that store large amounts of glycogen?
1. liver
2. muscle
Why are fats the most efficient storage molecules?
stores more energy per carbon than other molecules
(note: their carbons are in a very reduced state)
Which enzymes in adipose tissue are hormone sensitive (e.g to glucagon)?
Lipases
How is glycerol modified to enter glycolysis
converted to PGAL
How are fatty acids modified to enter the CAC?
converted to acetyl CoA
(Note: every two carbons on a fatty acid are converted to a single acetyl CoA molecule)
What are fatty acids attached to while in the blood stream?
albumin
What is the process that converts fatty acid chains into molecules of acetyl CoA?
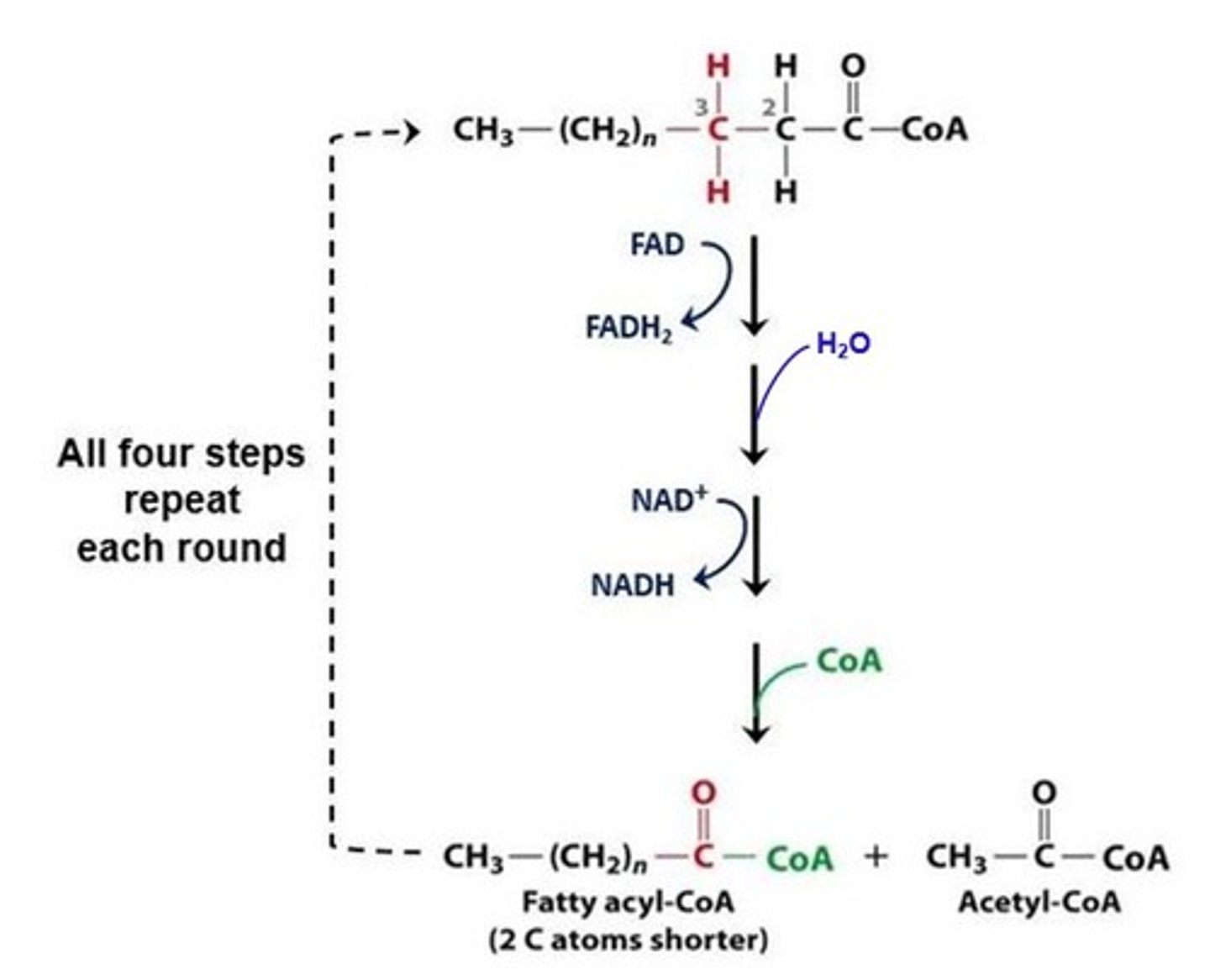
beta-oxidation
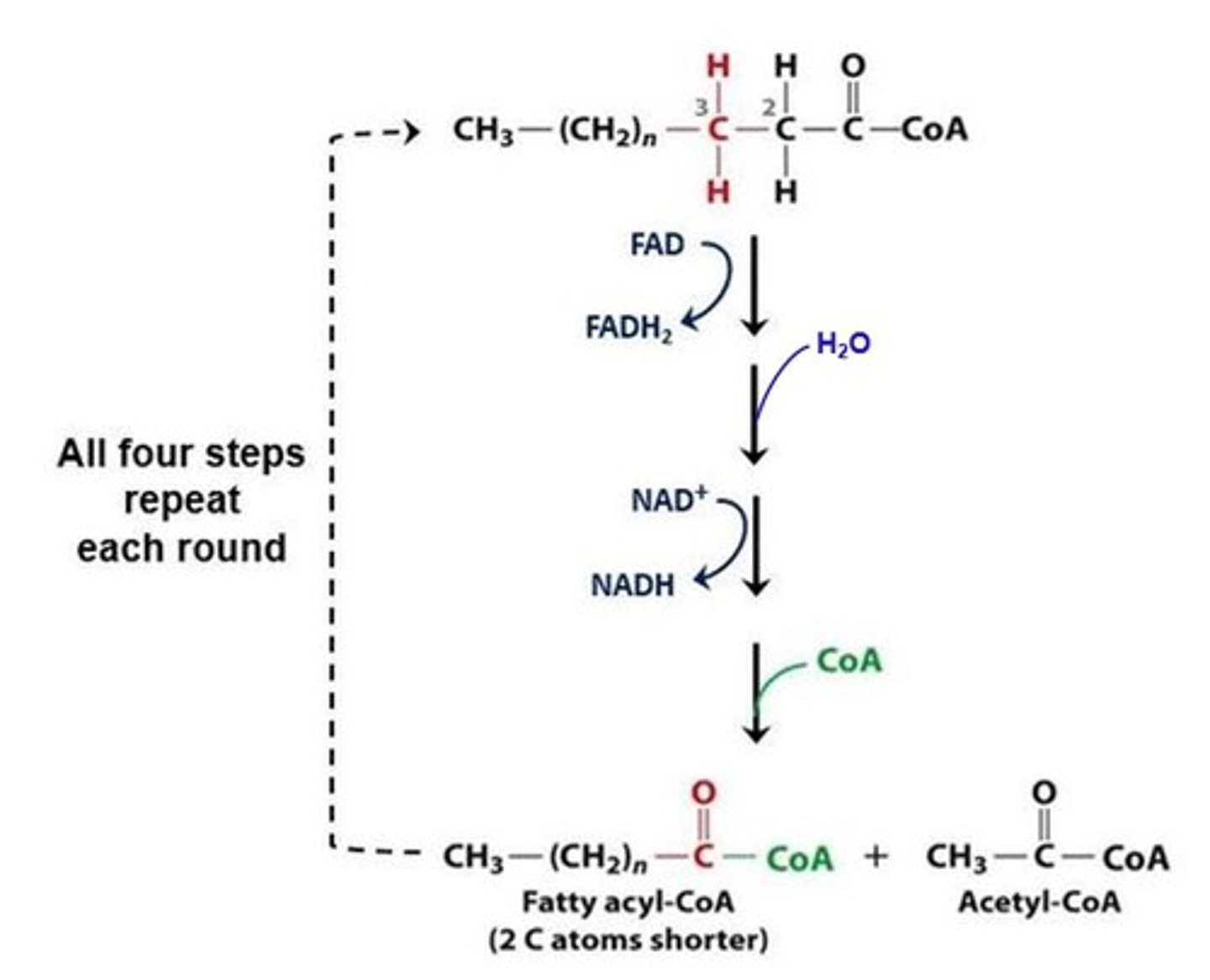
Where does beta-oxidation take place?
mitochondrial matrix
What is the energy investment to activate a fatty acid for catabolism?
2 ATP
For every molecule of acetyl CoA cleaved from a saturated fatty acid, what is the energy payoff for each cut?
1 NADH + 1 FADH2
(Note: every cut
produces these molecules)
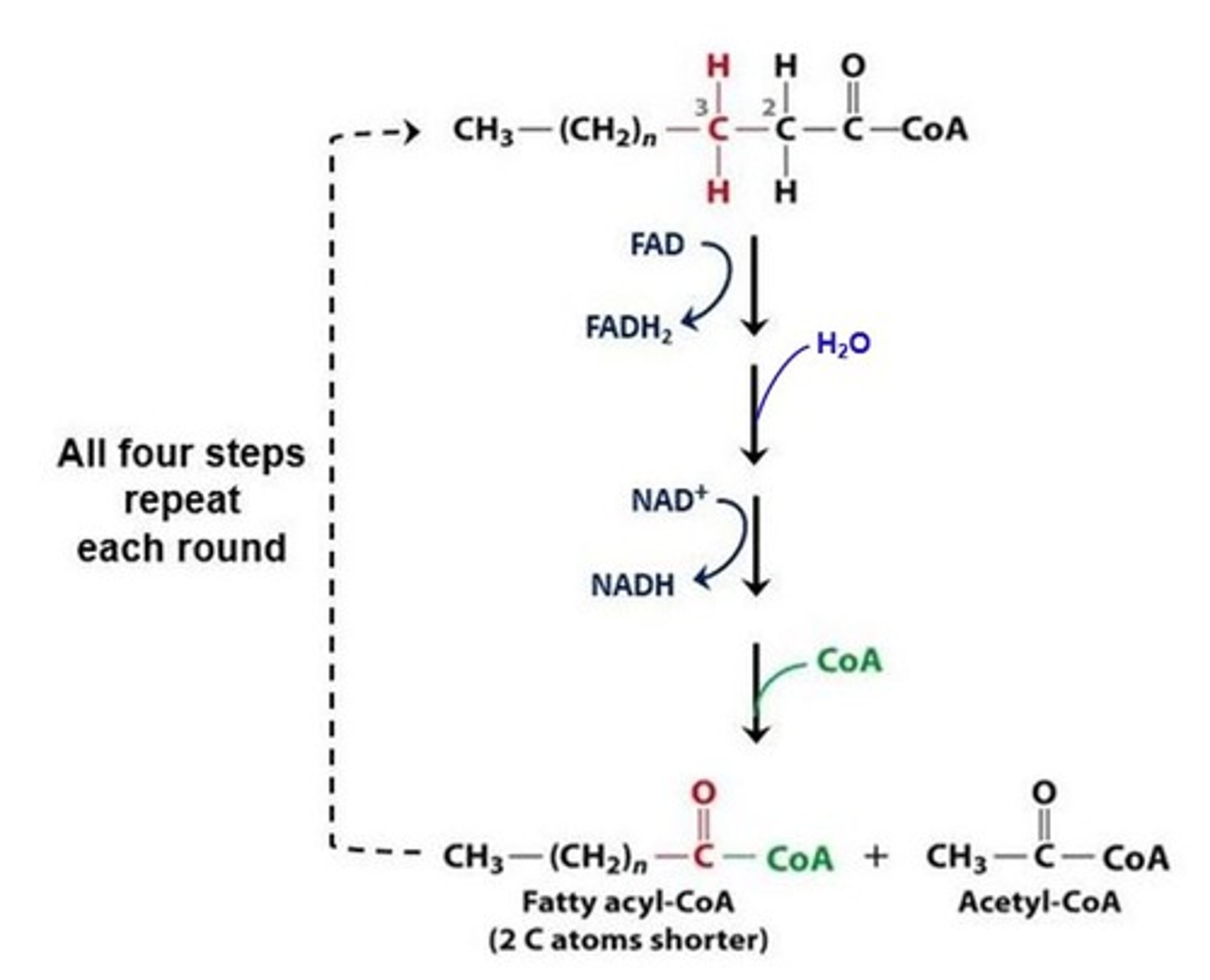
How many times will an 18 carbon saturated fatty acid be cut during beta-oxidation?
8
(Note: last cut splits a 4 carbon molecule into 2 acetyl CoA's)
How does the energy payoff of an unsaturated fatty acid compare to a saturated fatty acid?
unsaturated fatty acids
produce 1 less FADH2
per double bond
(Note: can't use the
double bond forming
step in beta-oxidation)
Between meals, most lipids of plasma are in what form?
lipoproteins
(Note: chylomicrons are large lipoproteins)
Low-density lipoproteins (LDL) have a low density of which molecules?
proteins
(Note: high fat = unhealthy)
High-density lipoproteins (HDL) have a high density of which molecules?
proteins
(Note: low fat = healthy)
How are amino acids activated for catabolism?
most amino acids are deaminated in the liver
After deamination, how are amino acids further modified for catabolism?
converted to pyruvate, acetyl CoA, or other CAC intermediates
(Note: each amino acid is different)
Oxidative deamination removes which molecule directly from amino acids?
ammonia
(Note: toxic to vertebrates)
How do most aquatic vertebrates and invertebrates excrete ammonia?
excrete it directly
How do insects, birds, and reptiles excrete ammonia?
convert it to uric acid
How do mammals, sharks, and most amphibians excrete ammonia?
convert it to urea