Core Practical 8: Calculate the enthalpy change for the thermal decomposition of potassium hydrogencarbonate
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
10 Terms
Overview:

Procedure:

Learning Tips:
the heat capacity of the final solution can be assumed to be the same as the heat capacity of the water. The volume of water made in the reaction is so small it can be ignored.
for exothermic reactions, the enthalpy change is negative
beware of the incorrect use of signs. For any enthalpy change it is crucial that you include a sign when you state the value but make sure it’s the correct one.
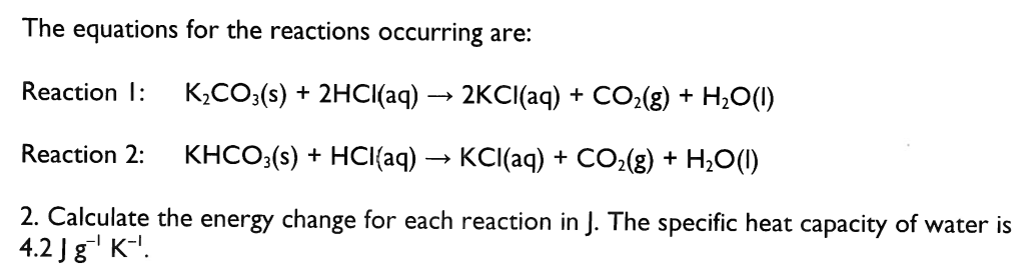
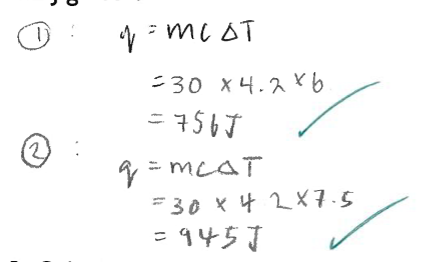
Calculate the enthalpy change for each reaction in kJmol^-1. Assume that hydrochloric acid is in excess.
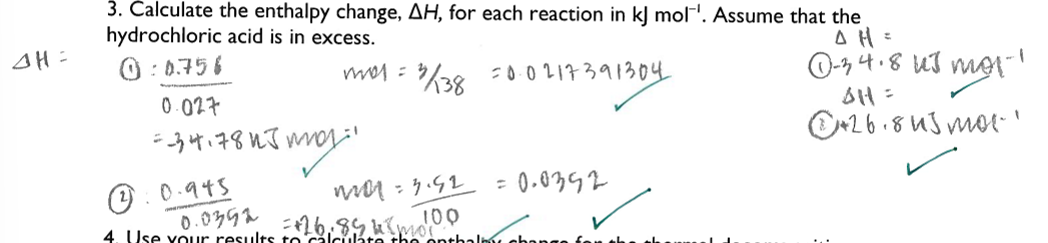
Use your results to calculate the enthalpy change for the thermal decomposition of potassium hydrogen carbonate:
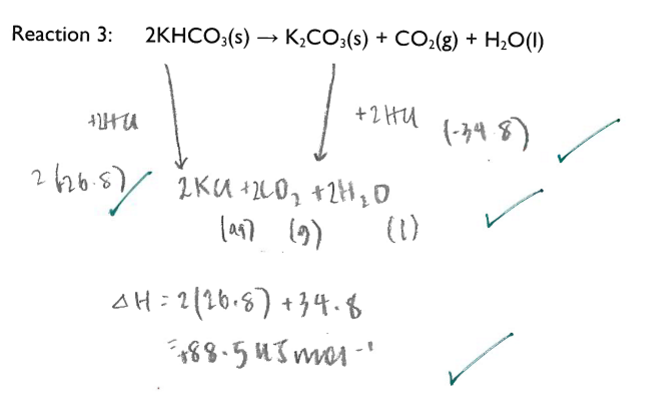
Why is it not possible to measure the enthalpy change for the decomposition of potassium hydrogencarbonate directly?
impossible to measure the temperature change during the reaction as heat is supplied to the reactants throughout the reaction
Show that hydrochloric acid is in excess in both reactions.

Draw energy level diagrams for reactions 1, 2 and 3.
Explain why the reactions are conducted in a polystyrene cup rather than a glass beaker.
polystyrene is a good insulator hence using this material instead of glass reduces the amount of heat loss to the surroundings