Dibartola Chapter 2: Applied Renal Physiology
1/183
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
184 Terms
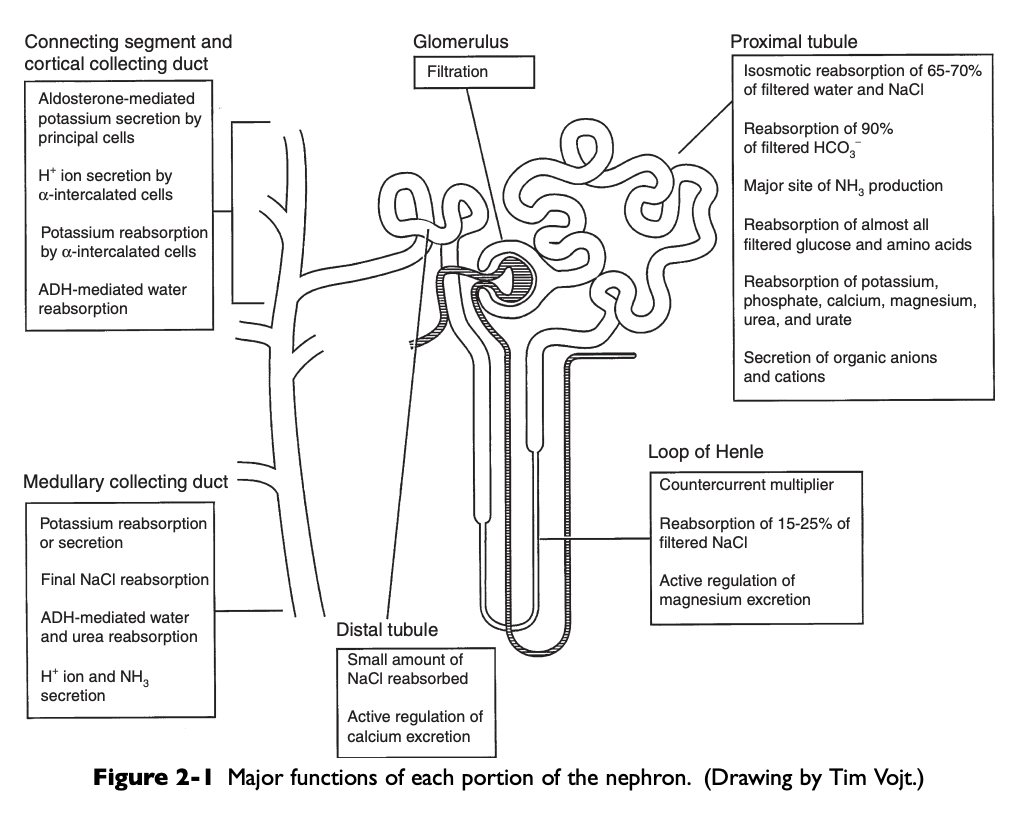
Major Functions of Each Portion of the Nephron Diagram
Renal Clearance
The volume of plasma that contains the amount of the substance excreted in the urine in 1 minute, the volume of plasma that must be filtered each minute to account for the amount of the substance appearing in the urine each minute under steady state conditions
Standard Renal Clearance Formula
Standard clearance formula: UxV/Px
Ux - the concentration of the substance in urine
V - urine flow rate
Px - the concentration of the substance in plasma
What are the three components of the glomerular capillary wall or filtration barrier?
Capillary endothelium
Basement membrane
Visceral epithelium
Structure of the Glomerulus
Glomerulus consists of a capillary bed interposed between the afferent and efferent arterioles
Capillary endothelium of the glomerulus is fenestrated by openings 50-100 nm in diameter
These openings exclude cells from the ultrafiltrate, but macromolecules are not restricted based on size
Luminal surface of the endothelium is covered by negatively charged sialoglycoproteins that contribute to the charge selectivity of the filtration barrier
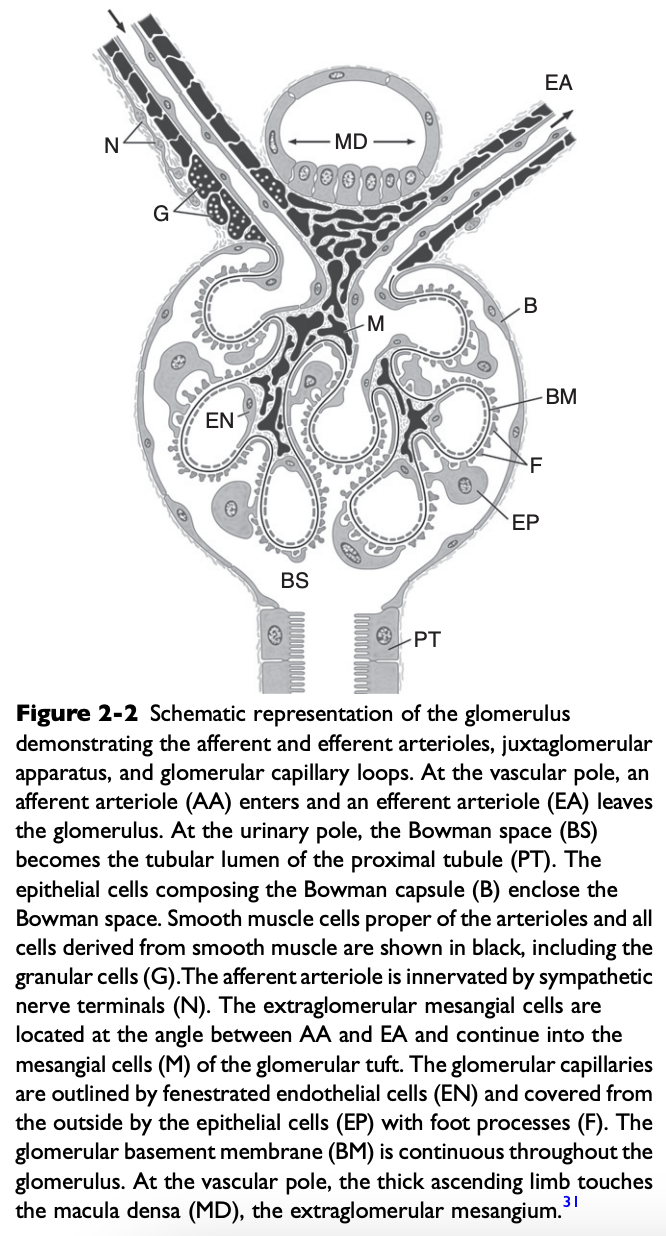
What is the glomerular basement membrane composed of?
Lamina rara interna on the endothelial side
Central lamina densa
Lamina rara externa on the epithelial side
Lamina Rara Interna and Lamina Rara Externa
Lamina rare interna and lamina rara externa contain polar noncollagenous proteins that contribute to the negative charge of the filtration barrier
Lamina Densa
Lamina densa contains nonpolar collagenous proteins that contribute primarily to the size selectivity of the filtration barrier
Filtration barrier is permeable to molecules with effective molecular radii less than 2 nm and impermeable to those with radii greater than 4 nm
Visceral Epithelial Cells or Podocytes
Visceral epithelial cells or podocytes constitute the outermost portion of the filtration barrier
Cover the glomerular basement membrane and glomerular capillaries on the urinary side of the barrier with their primary and interdigitating secondary foot processes
Filtration slits, 10-30 nm in width, are located between the secondary foot processes
Podocytes are phagocytic and may engulf macromolecules trapped by the filtration slits
Have a negatively charged sialoglycoprotein coat that contributes to the charge of selectivity of the filtration barrier
Believed to synthesize the glomerular basement membrane
Mesangium
Not part of the filtration barrier
Is a stabilizing core of tissue that forms an anchor for the glomerulus at the vascular pole and along the axes of the capillary lobules
Mesangial cells are in contact with the basement membrane in areas where there is no capillary endothelium
Extraglomerular mesangium fills the space between the macula densa and the glomerular arterioles and constitutes part of the juxtaglomerular apparatus (JGA)
Mesangial cells contain microfilaments that can contract in response to specific hormones (e.g. angiotensin II), altering the surface area available for filtration
Also synthesize prostaglandins that contribute to renal vasodilation
Contains macrophages that can clear filtration residues from the mesangial space by phagocytosis
What is the glomerular capillary wall selective for?
The glomerular capillary wall is a size-selective and charge-selective barrier to filtration
What gives the glomerular capillary wall its size selectivity?
Size selectivity primarily in the lamina densa of glomerular basement membrane
Generally excludes molecules with radii greater than 4 nm
What gives the glomerular capillary wall its charge selectivity?
Charge selectivity resides in the negatively charged sialoglycoproteins (e.g. laminin and fibronectin) and peptidoglycans (e.g. heparan sulfate) of the capillary endothelium, lamina rara externa, and visceral epithelium
Negatively charged macromolecules experience greater restriction to filtration than neutral ones
Positively charged macromolecules experience less restriction to filtration than neutral ones
Glomerular Filtration Rate
Total filtration rate of both kidneys and represents the sum of hte single-nephron glomerular filtration rates (SNGFRs) of all nephrons
Superficial Cortical Nephrons
Short loops of Henle with little or no penetration into the renal medulla
Tend to excrete relatively more solute and water
Juxtamedullary Nephrons
Long loops of Henle that penetrate the inner medulla
Tend to conserve solute and water
Glomerular Ultrafiltrate
A protein-free ultrafiltrate of plasma containing water and all of the cyrstalloids of plasma in concentrations similar to those in plasma
Single-Nephron Glomerular Filtration Rate Formula
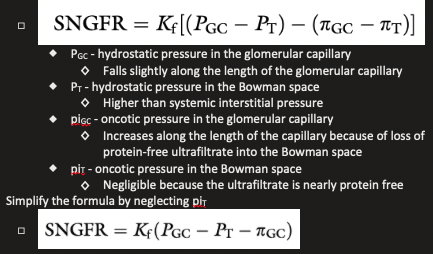
What accounts for the higher rate of filtration in the glomeular capillaries than in systemic capillaries?
If average pressure are considered alone, net filtration pressure in the glomerulus is approximately 15 mmHg, which is similar to systemic capillaries
The fact that GFR is so much higher than the movement of fluid across systemic capillaries is explained by different values for Kf
Ultrafiltration constant, Kf, is dependent on the surface area available for filtration and the permeability per unit area of capillary to crystalloids and water
In the glomerulus, the surface area available for filtration is much greater than that found in the capillary beds of skeletal muscle and the unit permeability of the glomerular endothelium is more than 100 times that of skeletal muscle capillaries
The much higher value for Kf in glomerular capillaries than in systemic capillaries accounts for the much higher rate of filtration
Kf is not constant and can change as a result of disease and in response to hormones that cause mesangial cells to contract (e.g. angiotensin II)
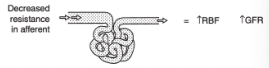
Effect of Changes in Resistance in the Afferent Arterioles
Leads to parallel changes in GFR and renal blood flow
Effect of Changes in Resistance in the Efferent Arterioles
Lead to divergent changes in GFR and renal blood flow
Effect of Decreased Resistance in the Afferent Arteriole on Renal Blood Flow and GFR
Increased renal blood flow
Increased GFR
Effect of Increased Resistance in the Afferent Arteriole on Renal Blood Flow and GFR
Decreased renal blood flow
Decreased GFR
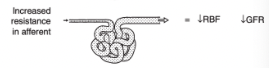
Effect of Decreased Resistance in the Efferent Arteriole on Renal Blood Flow and GFR
Increased renal blood flow
Decreased GFR
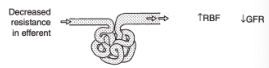
Effect of Increased Resistance in the Efferent Arteriole on Renal Blood Flow and GFR
Decreased renal blood flow
Increased GFR
Effect of Acetylcholine on the Afferent Arteriole
Relax
Effect of Nitric Oxide on the Afferent Arteriole
Relax
Effect of Dopamine on the Afferent and Efferent Arterioles
Relaxes both
Stimulation of dopaminergic receptors causes afferent and efferent vasodilation and increased renal blood flow with little change in GFR at low concentrations of dopamine
Effect of Bradykinin on the Afferent Arteriole
Relax
Effect of Prostacyclin on the Afferent Arteriole
Relax
Effect of Prostaglandin E2 on the Afferent Arteriole
Relax
Effect of Prostaglandin I2 on the Afferent Arteriole
Relax
Effect of Acetylcholine on the Efferent Arteriole
Relax
Effect of Nitric Oxide on the Efferent Arteriole
Relax
Effect of Bradykinin on the Efferent Arteriole
Relax
Effect of Prostacyclin on the Efferent Arteriole
Relax
Effect of Prostaglandin E2 on the Efferent Arteriole
No effect
Effect of Prostaglandin I2 on the Efferent Arteriole
Relax
Effect of Norepinephrine on the Afferent and Efferent Arterioles
Constricts both
Efferent arteriolar constriction usually predominates
Renal blood flow decreases with minimal changes in GFR (i.e. filtration fraction increases)
Effect of Angiotensin II on the Afferent and Efferent Arterioles
Constricts both
Causes efferent more than afferent vasoconstriction
Renal blood flow decreases with minimal changes in GFR (i.e. filtration fraction increases)
Effect of Endothelin on the Afferent Arteriole
Constrict
Effect of Thromboxane on the Afferent Arteriole
Constrict
Effect of Vasopressin on the Afferent Arteriole
No effect
Effect of Endothelin on the Efferent Arteriole
Constrict
Effect of Thromboxane on the Efferent Arteriole
Constrict
Effect of Vasopressin on the Efferent Arteriole
Constrict
Result of Norepinephrine, Angiotensin II, and ADH on Prostaglandins
Norepinephrine, angiotensin II, and ADH cause vasoconstriction at the same time promoting the production of prostaglandins that cause vasodilation
These prostaglandins (PGE2 and PGI2) play an important role in maintaining RBF in hypovolemic states when angiotensin II and norepinephrine concentrations are increased
Effects of these prostaglandins are limited to the kidneys because they are rapidly metabolized in the pulmonary circulation
NSAIDs that inhibit generation of prostaglandins by the COX pathway may cause renal ischemia and acute renal insufficiency in hypovolemic patients
Effect on Kinins on Renal Blood Flow
Locally produced kinins cause vasodilation and favor redistribution of renal blood flow to the inner cortical nephrons
Mass Balance Equation if there is a Substance that is Filtered by the Glomeruli but Neither Reabsorbed nor Secreted by the Tubules

Renal Clearance of a Substance that is Neither Reabsorbed nor Secreted
Equal to GFR
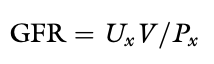
What is the laboratory standard for GFR determination?
Inulin clearance
What is used clinically to estimate GFR in the steady state?
Creatinine clearance
Creatinine Clearance
Creatinine is produced endogenously and excreted primarily by glomerular filtration so its clearance can be used to estimate GFR in the steady state
Requirements for determination of endogenous creatinine clearance are an accurately timed urine sample (usually 24 hours), determination of the patient's body weight, and measurement of serum and urine creatinine concentrations
In the dog and cat, creatinine is filtered by the glomeruli and neither reabsorbed nor secreted by the tubules
Values for endogenous creatinine clearance in the dog and cat are approximately 2-5 mL/min/kg
Horse 1-2 mL/min/kg
Renal Clearance Equation
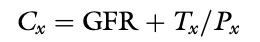
Clearance of a Substance Experiencing Net Reabsorption vs GFR
The clearance of a substance experiencing net reabsorption is less than GFR (Tx is negative)
Clearance of a Substance Experiencing Net Secretion vs GFR
The clearance of a substance experiencing net section is greater than GFR (Tx is positive)
Ratio of Clearance of a Substance to Inulin
The ratio of the clearance of a substance to inulin clearance gives an indication of the net handling of that substance by the kidneys
If ratio is less than 1.0, the substance experiences net reabsorption
If greater than 1.0, it experiences net secretion
What % of cardiac output do the kidneys receive?
25% or more
What are the major sites of resistance in the kidneys?
Afferent and efferent arterioles, with an approximately 80-90% decrease in perfusion pressure across this region of the renal vasculature
What % of renal blood flow is directed to the renal cortex?
More than 90%
What % of renal blood flow is directed ot the outer medulla?
Less than 10%
What % of renal blood flow is directed to the inner medulla?
2-3%
Autoregulation
The intrinsic ability of an organ to maintain blood flow at a nearly constant rate despite change in arterial perfusion pressure
In the kidneys, between perfusion pressures of 80 and 180 mmHg, GFR and RBF vary less than 10%
Flow Equation
Flow (Q) is equal to pressure (P) divided by resistance ( R )
Q = P/R
As pressure increases, flow can remain constant only if resistance increases proportionately
The site of this resistance change in the kidneys is the afferent arteriole
Effect of Anesthesia on Autoregulation
Impaired by anesthesia in proportion to the depth of anesthesia
Afferent arterioles are maximally dilated at MAPs of 70-80 mmHg and at lower pressures, GFR declines linearly with RBF (autoregulation is lost)
What are the physiologic mechanisms that contribute to autoregulation?
Myogenic mechanism
Tubuloglomerular feedback
Myogenic Mechanism
Based on the principle that smooth muscle tends to contract when stretched and relax when shortened
As the afferent arteriole is stretched by increased perfusion pressure, it constricts, thus limiting transmission of this increased pressure to the glomerulus and minimizing any change in glomerular capillary hydrostatic pressure and SNGFR
Represents a course control that operates with a delay of 1-2 seconds
Tubuloglomerular Feedback
Represents a local intrarenal negative feedback mechanism for individual nephrons
Increased sodium chloride concentration or transport in the distal tubule is sensed by the extraglomerular mesangial cells of the JGA as they monitor sodium chloride transport across the tubular cells of the macula densa
Transport of NaCl by the tubular cells of the macula densa requires functional NKCC2 (Na+, K+, 2 Cl cotransporter) and ROMK (potassium channel) in the luminal membranes and functional Na+, K+ ATPase in the basolateral membranes
Transcellular transport of NaCl causes generation of adenosine, which together with angiotensin II, causes afferent arteriolar constriction in the parent glomerulus
Afferent arteriolar constriction causes SNGFR to decrease, thus decreasing filtration and minimizing NaCl loss in the nephron
Effect occurs locally in the region of the juxtaglomerular interstitium
Represents fine control that operates with a 10-12 second delay
Renal Plasma Flow Equation
PAX - renal arterial plasma concentration of x
RPFA - arterial renal plasma flow (RPF)
PVx - renal venous plasma concentration of x
RPFV - venous RPF
Ux - urine concentration of x
V - urine flow
If we chose a substance that is completely removed from the blood in one pass through the kidneys, PVx is zero and RPF = Ux V /PAX
If the substance x is not metabolized and is not excreted by any organ other than the kidneys, its concentration in any peripheral vessel equals PAX
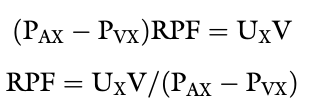
What is PAH clearance an estimate of?
Effective renal plasma flow
Fick Principle
The amount of a substance (V) removed by an organ is equal to the blood flow to the organ (Q) times the arteriovenous concentration difference of the substance in question (CA-CV)
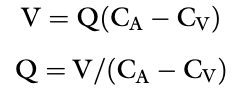
Renal Blood Flow Equation
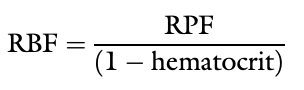
Filtration Fraction
The fraction of plasma flowing through the kidneys that is filtered into the Bowman space
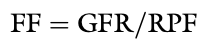
Reabsorption
In the kidneys, refers to movement of water and solutes from the tubular lumen to the peritubular interstitium
Secretion
In the kidney, refers to movement of water and solutes from the peritubular interstitium to the tubular lumen
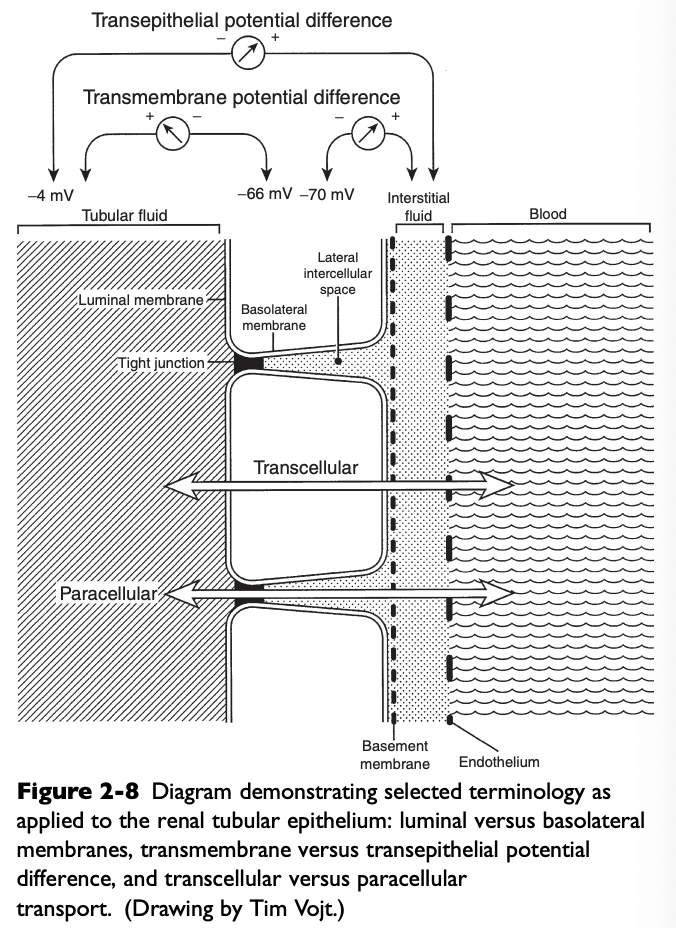
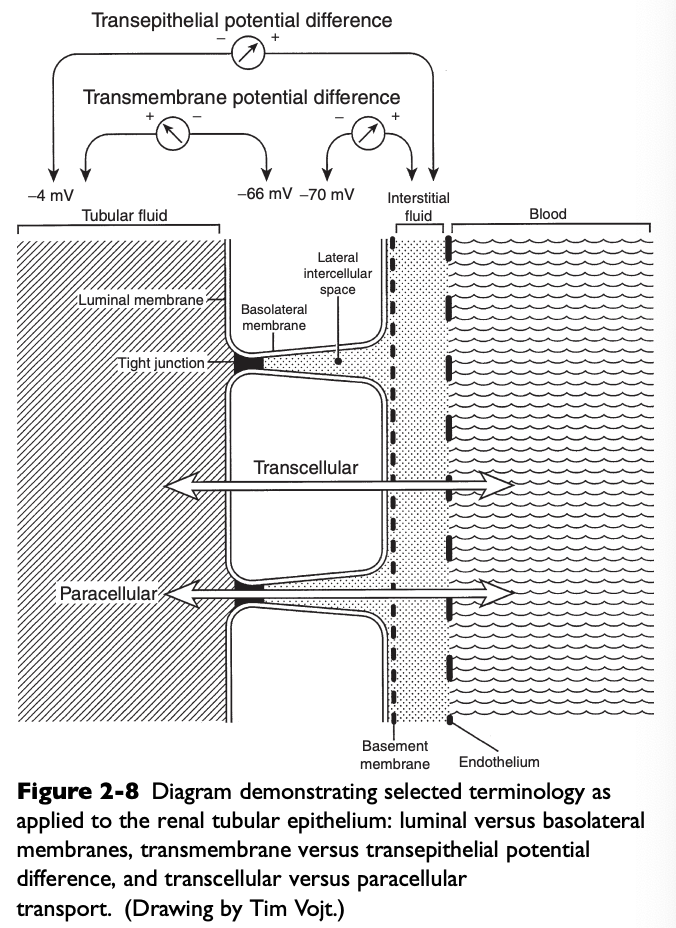
What do the luminal membranes of the kidney separate?
The cytoplasm of the tubular cell from the tubular fluid
What do the basolateral membranes of the kidney separate?
The cytoplasm of the tubular cell from the lateral intercellular space and peritubular interstitium
Transmembrane Potential Difference (PD)
The electrical PD between the outside and inside of the cell
Usually -60 to-70 mV (cell interior negative)
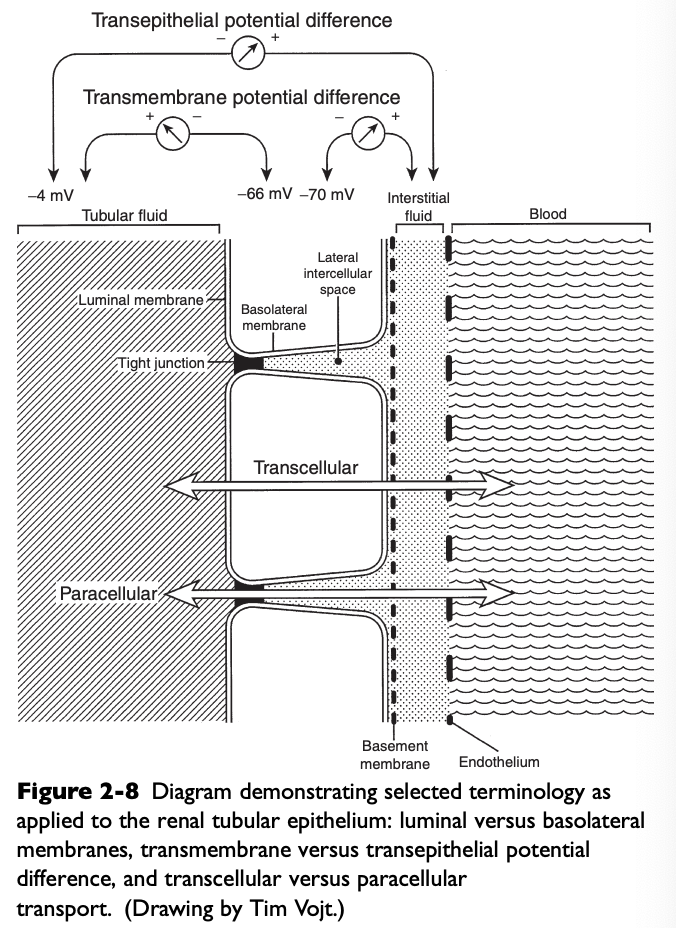
Transepithelial or Transtubular Potential Difference
The electrical PD between the tubular lumen and the peritubular interstitium, algebraic sum of the transmembrane PD between the tubular lumen and cell cytoplasm, and the transmembrane PD between the peritubular interstitium and cell cytoplasm
Only a few mV
Potential Difference Between the Tubular Lumen and Peritubular Lumen in the Early Proximal Tubule
The tubular lumen is a few mV negative relative to the peritubular lumen
Potential Difference Between the Tubular Lumen and Peritubular Interstitium in the Later Proximal Tubule
The tubular lumen is a few mV positive relative to the peritubular interstitium
Transepithelial Potential Difference in the Thick Ascending Loop of Henle
Lumen positive
Transepithelial Potential Difference in the Distal Tubule
Lumen negative
Paracellular Route
Movement of solutes and water between cells (i.e. from the tubular lumen to the lateral intercellular space across tight junctions connecting epithelial cells)
Allows movement of ions and large, nonpolar solutes by passive diffusion and solvent drag
Electrochemical, hydrostatic, and oncotic gradients are important driving forces for reabsorption by the paracellular route
Accounts for only 1% of the surface area available for reabsorption and 5-10% of water transport
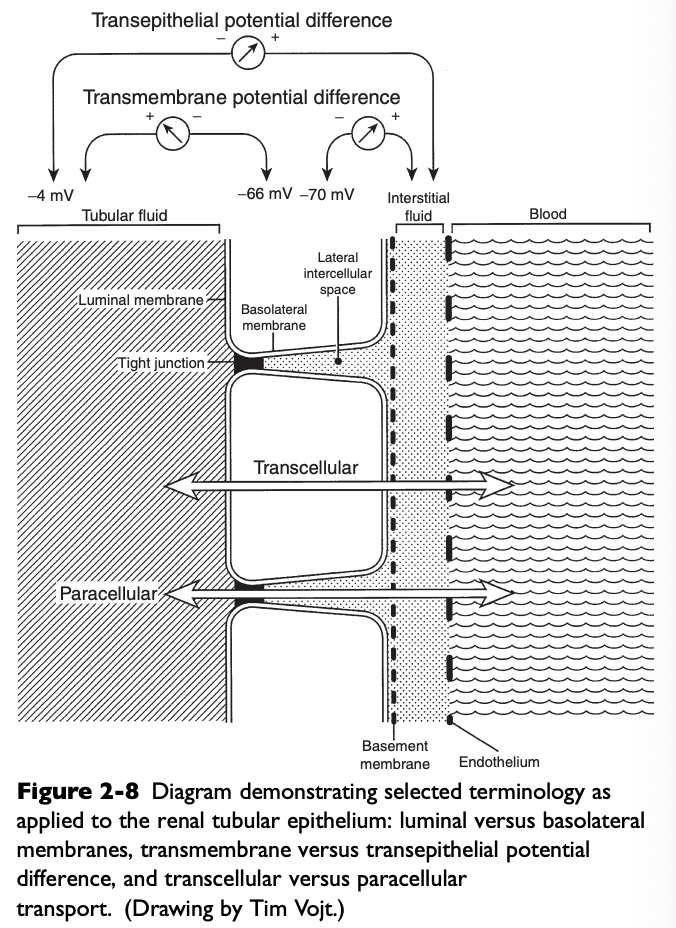
Transcellular Route
Movement of solutes and water through the cytoplasm of the tubular cells
Accounts for 99% of the available surface area and 90-95% of water transport
Both passive and active transport processes occur by this route
All active transport processes must occur by this route
Leaky Epithelial Cell Junctions
Proximal tubules
Do not generate large transepithelial concentration gradients, exhibit small transepithelial PD, and have high water permeability
Tight Epithelial Cell Junctions
Distal convoluted tubules, collecting ducts
Can generate large transepithelial concentration gradients, exhibit a large transepithelial PD, and have low basal water permeability

Water in the Proximal Tubule
In the proximal tubule, water follows solute reabsorption osmotically and is said to occur isosmotically (reabsorbed fluid has the same osmolality as extracellular fluid)
What fraction of water and solute reabsorption occurs in the proximal tubule?
2/3
What % of glucose, amino acids, and bicarbonate are reabsorbed in the early proximal tubules?
Almost 99% of glucose and amino acids and 90% or more of bicarbonate
What are the four types of transport processes that contribute to renal tubular reabsorption?
Passive diffusion
Facilitated diffusion
Primary active transport
Secondary active transport
Passive Diffusion
Movement of a substance across a membrane as a result of random molecular motion
Simple diffusion can take place directly through the lipid bilayer of the cell membrane and through hydrophilic protein channels embedded in the cell membrane
Simple diffusion requires no expenditure of metabolic energy
Rate of transfer of solute is dependent on the permeability characteristics of the membrane, the electrochemical gradient, and the hydrostatic pressure across the membrane
Rate of diffusion is linearly related to the concentration of the diffusing solute
No maximal rate of transfer (Vmax)
Not a saturable process because a carrier is not involved
Facilitated Diffusion
Movement of a substance across a membrane down its electrochemical gradient after binding with a specific carrier protein in the membrane
The carrier protein binds the substance to be transported at one side of the cell membrane, undergoes an conformational change that causes translocation of the substance across the cell membrane, and releases the substance on the other side of the membrane
Saturable process characterized by a maximal rate of transfer (Vmax) because a carrier is involved
The carrier has structural specificity and affinity for the substance transported and the process is subject to competitive inhibition
Does not directly require metabolic energy
Transfer may occur in either direction across the membrane
Primary Active Transport
Movement of a substance across a membrane in combination with a carrier protein but against the electrochemical gradient
Requires metabolic energy, supplied by hydrolysis of ATP
Saturable process characterized by a Vmax
Subject to metabolic (e.g. cellular oxidative poisons) and competitive inhibition
Examples
Na+, K+ ATPase in basolateral membranes of tubular cells throughout the nephron
H+ATPase in luminal membranes of tubular cells throughout the nephron
H+, K+ATPase in luminal membranes of a-intercalated cells in the collecting ducts
Secondary Active Transport
Movement of two substances across a membrane after combination with a single carrier protein
Uphill (against a concentration gradient) transport of one substance is linked to the downhill (down an electrochemical gradient) transport of another substance
Saturable
Demonstrates structural specificity and affinity of the carrier for the substances transported
May be competitively inhibited
Uphill transport occurs without direct input of metabolic energy, substance transported uphill said to experience secondary active transport
Metabolic energy for secondary active transport at the luminal membranes comes from the primary active transport of sodium out of the tubular cell at the basolateral membrane by Na+, K+ATPase
Cotransport
Transported substances are moving in the same direction across the membrane
Countertransport
Transported substances are moving in opposite directions across the membrane
Pinocytosis
Uptake by cells of particles too large to diffuse through the cell membrane
Solvent Drag
Process whereby water moving across an epithelium by osmosis can drag dissolved solutes along with it
Morphology of the Proximal Tubule
Brush border of the luminal surface of the proximal tubular cells consists of microvilli, which increase surface area, and lateral cellular interdigitations, which increase the surface area of the basolateral membranes
Mitochondria supply energy via ATP for active transport
The most proximal segments of the proximal tubule ultrastructurally are the most complex and suited for the mechanisms of solute transport
The morphologic complexity decreases along the length of the proximal tubule