Topic 3 - Chemical Changes✅
1/66
Earn XP
Description and Tags
love my life! not. if watching lando norris win a wdc before charles leclerc doesnt kill me, electrolysis will
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
67 Terms
What are acids in solutions sources of
Hydrogen ions
What are alkalis in solutions sources of
Hydroxide ions
What is the pH of a neutral solution
7
What is the pH of acidic and alkaline solutions compared to neutral solutions
• Acidic solutions = lower than 7
• Alkaline solutions = higher than 7
What the effect of acid and alkali for litmus solution
• Alkaline = blue
• Acidic = red
What is the effect of acid and alkali on blue litmus paper
• Stays blue in alkali
• Goes red in acidic
What is the effect of acid and alkali on red litmus paper
• Goes blue in alkaline
• Stays red in acidic
What the effect of acid and alkali for methyl orange
• Alkaline = yellow
• Acidic = red
What is the effect of acid and alkali for litmus solution
• Alkaline = blue
• Acidic = red
What the effect of acid and alkali for phenolphthalein
• Alkaline - pink
• Acidic = colourless
How does concentration of hydrogen ions relate to pH
The higher the concentration of hydrogen ions in acidic solution, the lower the pH
How does concentration of hydroxide ions relate to pH
The higher the concentration of hydroxide ions in alkaline solution, the higher the pH
How is hydrogen ion concentration proportionate to pH
• As H+ ion concentration increases by a factor of 10, the pH decreases by 1.
• e.g when H+ increases from 10 to 1000, pH decreases by 1
Core practical METHOD - investigate the change in pH on adding calcium hydroxide to hydrochloric acid
1. Add 25cm of dilute hydrochloric acid to a beaker
2. Add some universal indicator
3. Compare initial colour to a pH colour chart and record pH
4. Add calcium hydroxide one spatula at a time
5. Stir and record pH
6. Keep adding calcium hydroxide until the pH remains constant
How can you improve the accuracy of Core practical "investigate the change in pH on adding calcium hydroxide to hydrochloric acid"
• Use a volumetric pipette instead of a measuring cylinder
• Use a pH probe instead of universal indicator
Explain the term dilute
Lesser amount of substance in a given volume of solution
Explain the term concentrated
Larger amount of substance in a given volume of a solution
Explain the term strong acid
• Fully dissociates in aqueous solution
• (fully breaks down to release H+ ions)
Explain the term weak acid
• An acid that partially dissociates in aqueous solution
• (so less H+ ions are released)
What is a base
Any substance that reacts with an acid to form a salt and water only
What are alkalis
Soluble bases
General reaction of aqueous acids with metals
Acid + metal = salt + hydrogen
General reaction of aqueous acids with metal oxides
Metal oxide + acid = salt + water
General reaction of aqueous acids with metal hydroxides
Metal hydroxide + acid = salt + water
General reaction of aqueous acids with metal carbonates
Metal carbonate + acid = salt + water + carbon dioxide
Describe the test for hydrogen
• Place a light splint in a test tube of gas
• If the gas is hydrogen there will be a squeaky pop
Describe the test for carbon dioxide
• Bubble the gas through limewater
• If carbon dioxide is present, the limewater goes cloudy
What is a neutralisation reaction
When an acid and alkali neutralise each other to produce a salt and water
What is an acid-alkali neutralisation
A reaction in which hydrogen (H+) ions from the acid react with hydroxide (OH-) ions from the alkali, to form water
Explain why, if soluble salts are prepared from an acid and an insoluble reactant, an excess of the reactant is added
So the volume of acid reacts completely
Explain why, if soluble salts are prepared from an acid and an insoluble reactant, the excess reactant is removed
So you are left with just a salt and water
Reactant filtered out
Explain why, if soluble salts are prepared from an acid and an insoluble reactant, the solution remaining is only salt and water
As the acid has reacted completely and reactant has been filtered out.
(If a carbonate, carbon dioxide given off to the atmosphere)
Explain why, if soluble salts are prepared from an acid and a soluble reactant, a titration must be used
• Both reactants are soluble, so if one is in excess, you won't be able to remove it easily from the mixtures.
• So you need exact volumes, which can be measured using titration.
Explain why, if soluble salts are prepared from an acid and a soluble reactant, the acid and the soluble reactant are then mixed in the correct proportions
So there is no left over acid or alkali.
Explain why, if soluble salts are prepared from an acid and a soluble reactant, the solution remaining, after reaction, is only salt and water
Because the acid and alkali have completely neutralised.
Core practical METHOD - investigate the preparation of pure, dry, hydrated copper sulfate
1. Using a measuring cylinder, measure 25cm of sulfuric acid into a beaker
2. Place beaker into water bath
3. Add copper oxide to the acid, 1 spatula at a time, stirring between
4. Continue adding copper oxide until it is in excess
5. Put a piece of filter paper in a funnel over a beaker
6. Pour the solution through the funnel to remove excess copper sulfate
7. Pour the filtrate into an evaporating basin
8. Place on a tripod with gauze, and heat with a Bunsen burner
9. When all the water has evaporated, turn off the heat and leave to dry
10. Blue copper sulfate crystals will remain in the basin
Why is copper oxide added in excess for this core practical?
So that all of the sulfuric acid reacts
Describe the steps to carry out an acid-alkali titration
1. Wash burette using acid and water
2. Fill burette to 100cm
3. Add 25cm of alkali into a conical flask
4. Add some phenolphthalein as an indicator
5. Add acid from burette to the alkali until the phenolphthalein goes colourless
6. The titre (volume of acid needed to neutralise the acid) is the difference between the first and second reading on the burette
7. Repeat to gain more precise results
After carrying out an acid-alkali titration how do you prepare a pure, dry salt
• Warm the salt solution to evaporate the water
• Leave to dry
• Crystals form
Solubility rules for nitrates
All nitrates are soluble in water
Solubility rules for chlorides
Common chlorides are soluble in water, except silver and lead
Solubility rules for sulfates
Common sulfates are soluble in water except lead, barium and calcium
Solubility rules for carbonates
Common carbonates are insoluble except sodium, potassium, ammonium
Solubility rules for hydroxides
Common hydroxides are insoluble except sodium, potassium, ammonium
Solubility rules for common salts
All common sodium, potassium and ammonium salts are soluble in water
When do you know if a precipitate will be formed when named solutions are mixed together
• Use the solubility rules to determine if salts formed are soluble or insoluble
• Insoluble salts will form as precipitate
Describe the method used to prepare a pure, dry sample of an insoluble salt
• mix the two solutions needed to form the salt
• filter the mixture using filter paper, which the insoluble salt will be left on
• wash the salt using distilled water
• leave the salt to dry on filter paper
• (water will evaporate, speed this process up by drying it in an oven)
What are electrolytes
· Ionic compounds that are molten or dissolved in water
· When an ionic substance is melted or dissolved in water the ions are free to move
What is electrolysis
· passing an electric current through a solution to cause a chemical reaction that splits the substance into its elements.
· Passing a current through a solution means that the solution can be broken down into elements
Explain the movement of ions during electrolysis
· The positively charged ions (cations) moved to the negatively charged electrode (cathode).
· Negatively charged ions (anions) move to the positive electrode (anode)
What happens during the electrolysis of copper chloride solution
Cathode
• Cu+ ions go to the cathode
• Cu is produced
Anode
• Cl- ions go the anode
• Cl2 is produced
What happens during the electrolysis of sodium chloride
Cathode
• H+ ions go to the cathode
• H2 is produced
Anode
• Cl- ions go to the anode
• Cl2 is produced
What happens during the electrolysis of sodium sulfate
Cathode
• H+ ions go the cathode
• H2 is produced
Anode
• OH- ions go to the anode
• O2 is produced
What happens during the electrolysis of water acidified with sulfuric acid
Cathode
• H+ goes to the cathode
• H2 is produced
Anode
• OH- goes to the anode
• O2 is produced
What happens during the electrolysis of molten lead bromide
Cathode
• Pb2+ goes to the cathode
• Pb is produced
Anode
• Br- goes to the anode
• Br2 is produced
Predict the products of electrolysis of other ionic compounds in the molten state
cathode
if the cation is less reactive than hydrogen, it is produced at the cathode
if it is more reactive than hydrogen, then H+ → H2
Anode
if it is not a halide then OH- → O2
if it is a halide then it is produced at the anode
Write half equations for the anode and cathode
Cathode X+ + e- → X
Anode X- → X + e-
What is OILRIG
· Oxidation is loss of electrons
· Reduction is gain of electrons
Which OILRIG occurs at the anode and which at the cathode and why
· Cathode = reduction because electrons are gained to obtain a neutral charge
· Anode = oxidation as there is a loss of electrons to obtain a neutral charge
Explain how electrolysis can be used to purify copper
|
Core practical METHOD - electrolysis with inert electrodes
1. Pour copper sulfate solution into a beaker to the half way point
2. Place two inert electrodes into the beaker
3. Attach to a power supply using crocodile clips and wires
4. Fill 2 test tubes with copper sulfate solution and place over electrodes
5. Turn on power
6. Record observations
7. Use a glowing splint to test for gas that has been collected in the test tubes
Diagram for electrolysis with inert electrodes
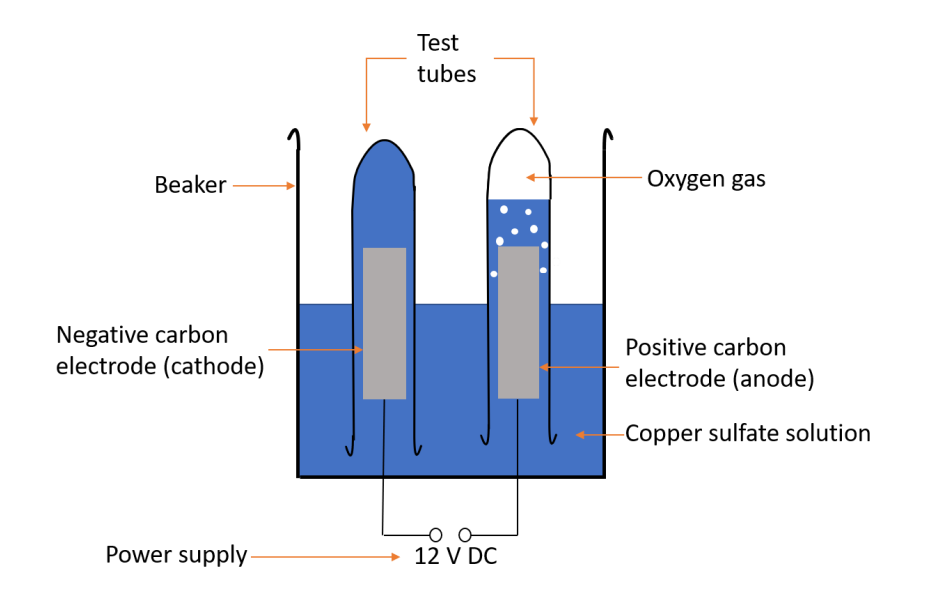
Results for inert electrodes (observation)
Negative electrode
• Pink solid forms
Positive electrode
• Bubbles of colourless gas
Results for inert electrodes (gas test)
Negative electrode
• None
Positive electrode
• Gas relights a glowing splint - so oxygen is produced
Core practical METHOD - electrolysis with copper electrodes
1. measure the mass of copper electrode and attach to the negative power supply
2. Measure mass of another copper electrode and attach to positive power supply
3. Pour copper sulfate solution into a beaker and place electrodes in the beaker
4. Turn on the power supply for 20 minutes
5. Remove each electrode and wash in distilled water
6. Measure the mass of both electrodes again
7. Repeat the steps with fresh electrodes and copper sulfate solution, but using a different current
Diagram for electrolysis with copper electrodes
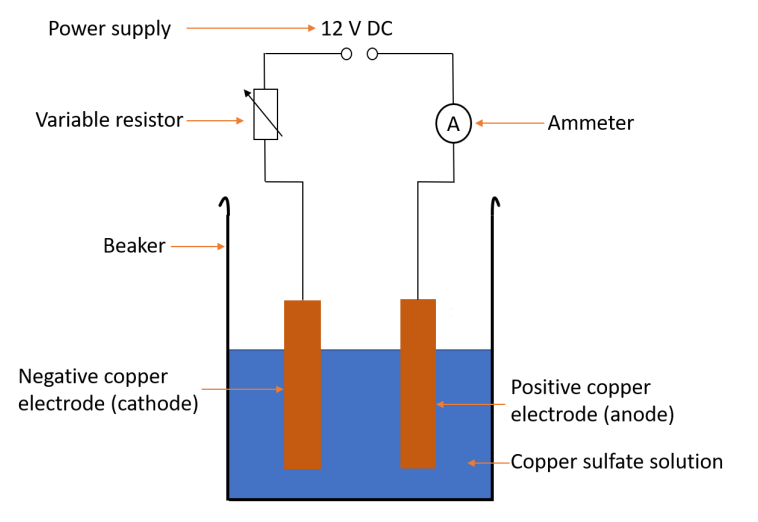
Results for electrolysis with copper electrodes
• The mass gained by the negative electrode is equal to the mass lost by the positive electrode
• Mass gained is proportional to current