Fundamentals of Spectroscopy & Applications of Spectroscopy (L02; chptrs 18 & 19)
1/37
Earn XP
Description and Tags
CHEM 310: Foundations of Analytical Chemistry
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
Spectroscopy
measuring the extent to which radiant energy (light) is absorbed by a chemical system as a function of wavelength of radiation (i.e. spectral scanning) as well as absorption measurements at a fixed wavelength
electromagnetic spectrum
various optical phenomenon are best interpreted in terms of the idea that light is propagated in the form of transverse waves
spectroscopy vs. spectrometry
spectroscopy = experiments with electromagnetic radiation
spectrometry = the application of spectroscopy so that there are quantifiable results
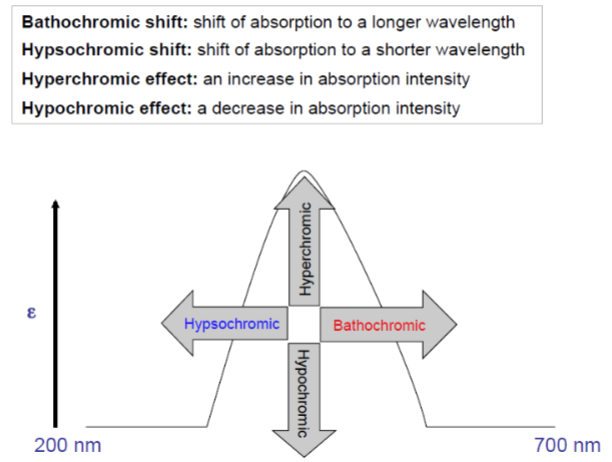
UV Spectroscopy: Terminology for absorptive shifts - Bathochromic shift
shift of absorption to a longer wavelength
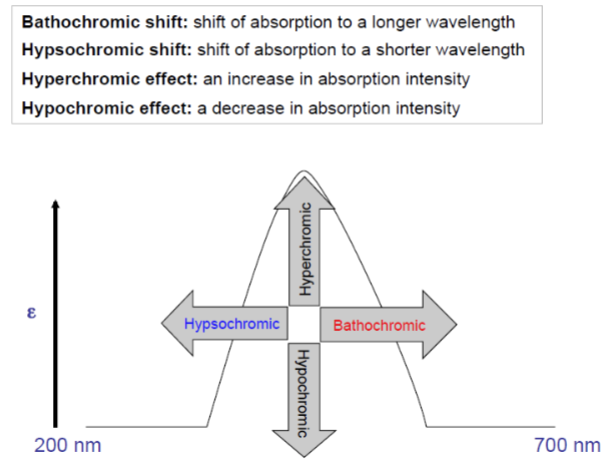
UV Spectroscopy: Terminology for absorptive shifts - hypsochromic
shift of absorption to a shorter wavelength
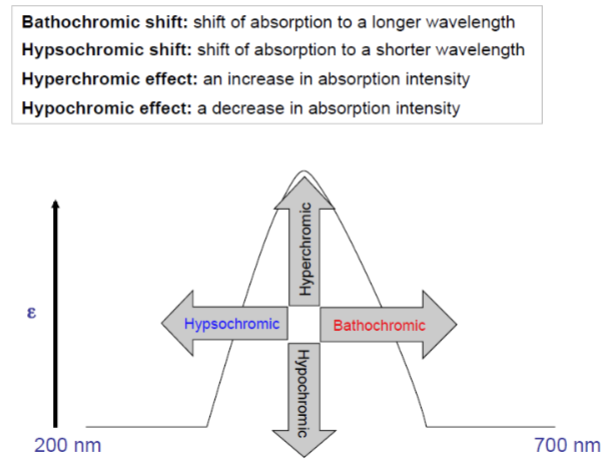
UV Spectroscopy: Terminology for absorptive shifts - hyperchromic effect
an increase in absorption intensity
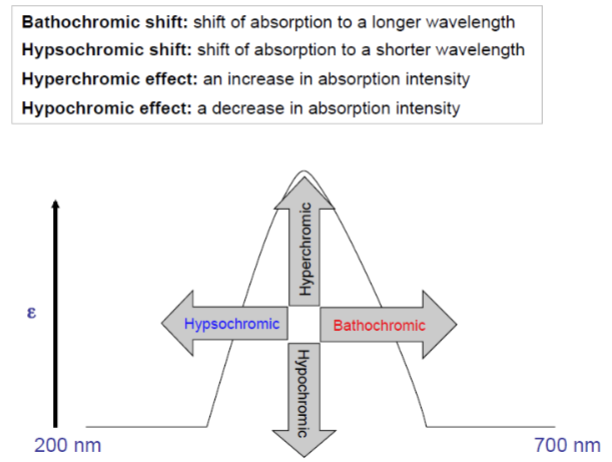
UV Spectroscopy: Terminology for absorptive shifts - hypochromic effect
a decrease in absorption intensity
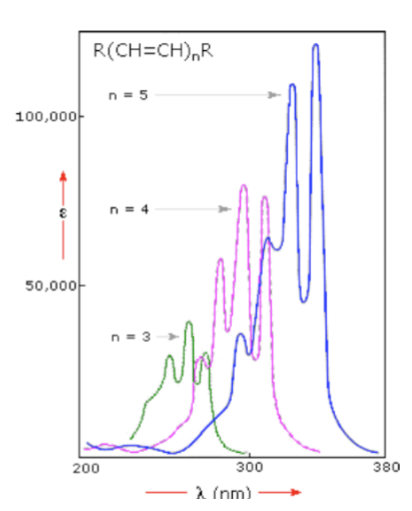
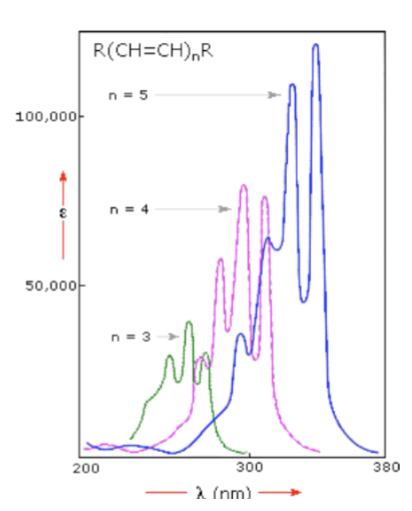
UV Spectroscopy: Conjugation Effects - the greater the conjugation…
the lower the energy required to induce electronic transitions (the longer the wavelength)
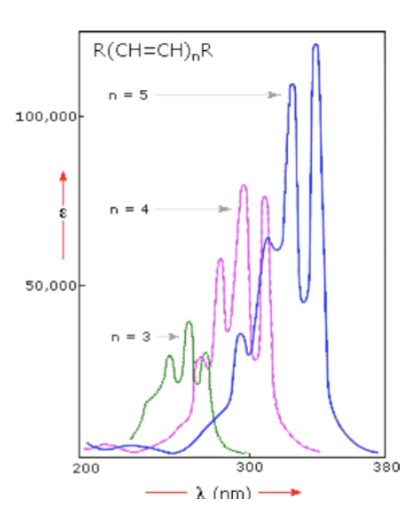
UV Spectroscopy: Conjugation Effects - lengthening conjugation will…
increase band intensity (and make the molar absorptivity greater)
UV Spectroscopy: Conjugation Effects - adding substituents may…
increase band intensity (and make the molar absorptivity greater) by a much smaller degree than lengthening conjugation would
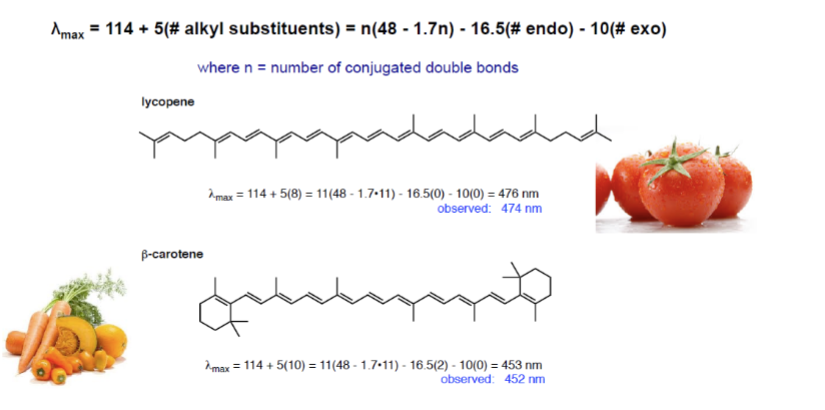
UV Spectroscopy: Fieser-Kuhn Rules for Dienes
used to reliably predict absorption wavelength in dienes, enone, and (to a lesser extent) aromatic systems
tip: watch some videos on YT about this because I don’t really know what this slide is trying to say
UV Spectroscopy: Fieser-Kuhn Rules for Polyenes
max wavelength = 114 + 5M + n(48-1.7n) - 16.5(# Rendo) - 10(# Rexo)
M = # alkyl substituents
n = # conjugated double bonds
Rendo = # rings with endocyclic double bonds
Rexo = # rings with exocyclic double bonds
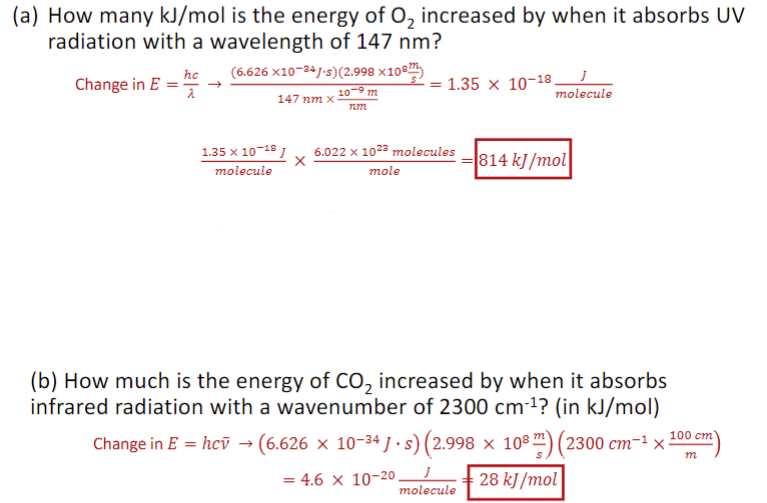
Photon Energies
each packet has an associated energy: Ephoton = hv = (hc) / 𝜆 = ℎ𝑐 ̅𝜈, where h = 6.626 × 10-34 J
Interaction of light with molecules: electronic energy, Eelec
absorption of UV and visible light increases electronic energy that causes electrons to be excited to higher energy levels
Interaction of light with molecules: vibrational energy, Evib
changed in vibrational energy are seen in the infrared region
Interaction of light with molecules: rotational energy, Erot
rotational transition requires relatively little energy and are induced by radiation of very low frequency (long wavelength) typically in the far-IR or microwave region
Light Absorption Process
In general, when light causes electronic transitions, vibrational and rotational transitions occur as well. Electronic absorption bands are usually broad because of many different vibrational and rotational transitions.
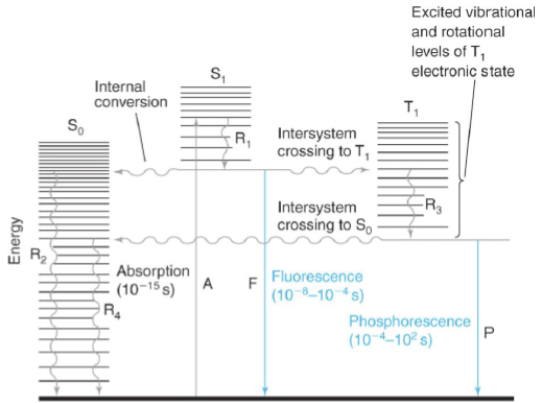
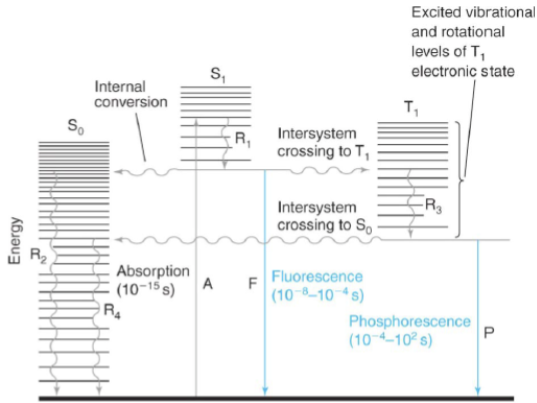
internal conversion
radiation-less transition between levels with the same spin states (i.e. Sn → S1 excited vibrational level)
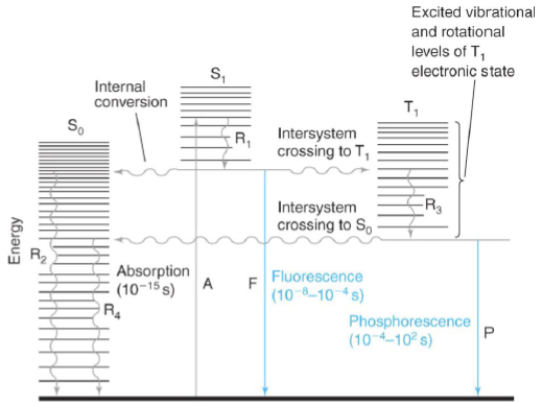
intersystem crossing
radiation-less transition between levels with different spin states (i.e. S1 → T1)
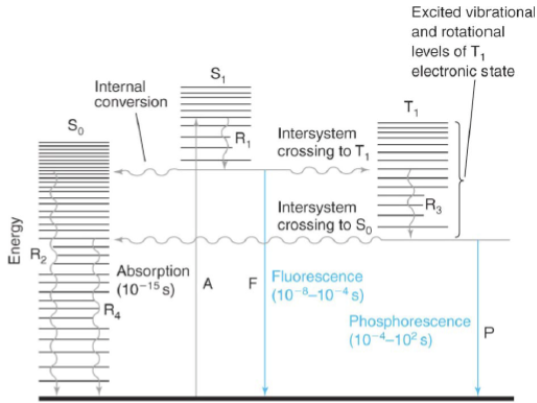
fluorescence
emission of a photon during a transition between levels with the same spin states (i.e. S1 → S0)
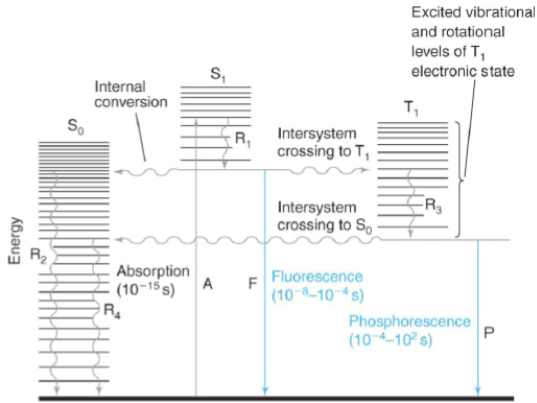
phosphorescence
emission of a photon during a transition between levels with different spin states (i.e. S1 → S0)
What are the basic parts of a (single beam) spectrometer?
Light source: Tungsten/Halogen lamp
Filter (Absorption filter/Interference filter)
Sample Holder
Detector = silicon photodiode (photoemmisive or photovoltaic cell)
Galvanometer
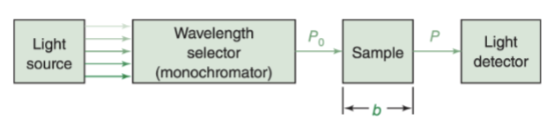
Application of single-beam spectrometry?
to obtain an absorption measurement in the visible region
What are the basic parts of a double-beam spectrometer?
Light source
Diffraction grafting
Slit
Beam splitter
Sample cell → mirror & mirror → reference cell
Beam splitter
Detector and computer
Chart recorder
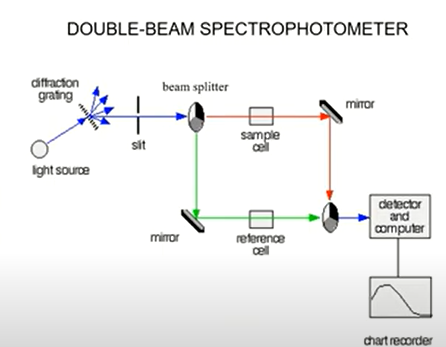
Application of double-beam spectrometry?
UV and Visible region (280-800 nm); obtains a photometric measurement, spectral scan, and kinetic measurement of two cells simultaneously
Single-beam Spectrometer vs. Double-beam Spectrometer
Light source is non-split light beam with high throughout of light vs. a split-into-two-fractions beam with low throughput of light
Used for simple structures and requires fewer components vs. complex structures and requires more components
Data quality: low reproducibility in the absorption measurement, so a less accurate result is obtained than what you’d get with a double-beam spectrophotometer
& the error due to the intensity radiation in double-beam is eliminated, which is a nice advantage
Transmittance (T)
the fraction, ranging from 0-1 (and usually referred to in %), of the original light that passes through the sample
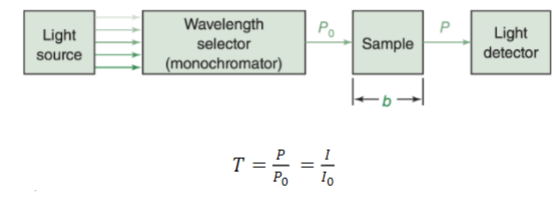
Absorbance (A)
is directly proportional to the concentration of the light absorbing molecular species.. in other words, it is directly proportional to the concentration of the sample
Why do we use blanks in a spectrometer?
they’re used to compensate for any absorbance originating from reagents or contamination
spectrophotometry, simply explained:
transmittance (the amount of light you see come out divided by the amount of light you put in) will decrease if you increase concentration to make your solution more opaque AND/OR have a wider (more volume) cuvette that the light has to pass through because light has to “bump into” more molecules of solvent as it passes through the cuvette
Beer-Lambert Law, simply explained
absorbance increases as your concentration increases, and transmittance increases as absorption decreases (T and A are inversely related)
Absorbance and Beer’s Law
𝐴 = 𝜀 b c
𝜀 = molar absorption coefficient (M-1cm-1) [aka extinction coef., molar absorptivity coef.] b = cell path length (usually 1 cm)*
𝑐 = molar concentration (mol/L)
𝐴 = − log 𝑇
𝑇 = 10logT = 10-A
![<p><span style="font-family: serif; color: #000000">𝐴 = 𝜀 b c</span><span style="color: #000000"><br></span><span style="font-family: serif; color: #000000">𝜀 </span><span style="font-family: sans-serif; color: #000000">= molar absorption coefficient (M<sup>-1</sup>cm<sup>-1</sup>) [aka extinction coef., molar absorptivity coef.]</span><span style="font-family: serif; color: #000000"> b </span><span style="font-family: sans-serif; color: #000000">= cell path length (usually 1 cm)*</span><span style="color: #000000"><br></span><span style="font-family: serif; color: #000000">𝑐 </span><span style="font-family: sans-serif; color: #000000">= molar concentration (mol/L)</span></p><p><span style="font-family: serif; color: #000000">𝐴 = − log 𝑇</span><span style="color: #000000"><br></span><span style="font-family: serif; color: #000000">𝑇 = 10<sup>log</sup><em><sup>T</sup></em> = 10<em><sup>-A</sup></em></span></p>](https://knowt-user-attachments.s3.amazonaws.com/880e87c0-09ab-4a7a-bacb-fd37bf4b9141.png)
Limitations of Beer’s Law in Absorbance
Applies to monochromatic radiation only
Works for dilute solutions, usually ≤ 0.01 𝑀𝑀
Absorbing species (i.e. the chromophore) does not participate in a concentration-dependent equilibrium
chromophore
the absorbing species
observed color of light is called the …
compliment of the color absorbed by the chromophore
When Beer’s Law Fails:
positive deviations: due to chemical effects
negative deviations: due to chemical effects, polychromatic light, or stray light
when your sample has a high concentration and the solute molecules have interacted with one another (because of the concentration-dependent chemical equilibrium, absorptivity changes with concentration)
Beer’s Law only applies to monochromatic light! It assumes that all light reaching the detector has passed through the sample. Scattered light may happen when you’re having solubility issues / free particles in your solutions / aggregation.
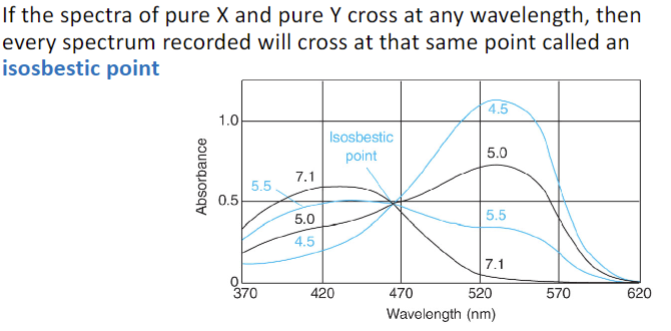
Isosbestic Point
when one absorbing species is converted into the other absorbing species